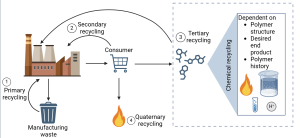
1 End of Life of Polymers
Depolymerization to Generate Useful Feedstocks
Madigan Petri
Introduction
Since Hermann Staudinger first proposed the possibility of polymerization in 19201 plastic manufacturing has grown exponentially into a $500 billion industry2. Plastics offer many advantages over non-petroleum-based materials like metal and glass because of their low cost, lightweight, durability, and inert qualities. Although plastic manufacturing has many sustainability challenges such as raw material origins, by-products (see Chapter 2), solvent/water usage, and energy usage, many consumer and corporate efforts target post-consumer plastic. Most synthetic polymers are bio-persistent because they resist degradation by microbes and bacteria. For that reason, plastic waste is a universal challenge in both environmental efforts and urban planning initiatives.
There are four main mechanisms or types of recycling currently in use to prevent post-consumer plastic from entering landfills, dubbed primary, secondary, tertiary, and quaternary recycling3 as seen in Figure 1. Primary recycling is the reuse of plastic materials without a sorting process. Generally, the same products are recycled into other products with identical materials. For example, a manufacturer collects manufacturing waste to reincorporate it into new products, such as shredding and melting. Secondary recycling is similar to primary but has a sorting process because the incoming material is usually mixed and post-consumer, such as home recycling. Both primary and secondary recycling typically use thermoplastics, or polymers that can be melted and reformed, because melting is a common method to create new products. Tertiary recycling is often called chemical recycling because the polymer is broken down at the chemical level to generate monomers, commercial chemicals, or fuel. Quaternary recycling is burning plastics to recover energy and reduce their mass and volume for landfill3.
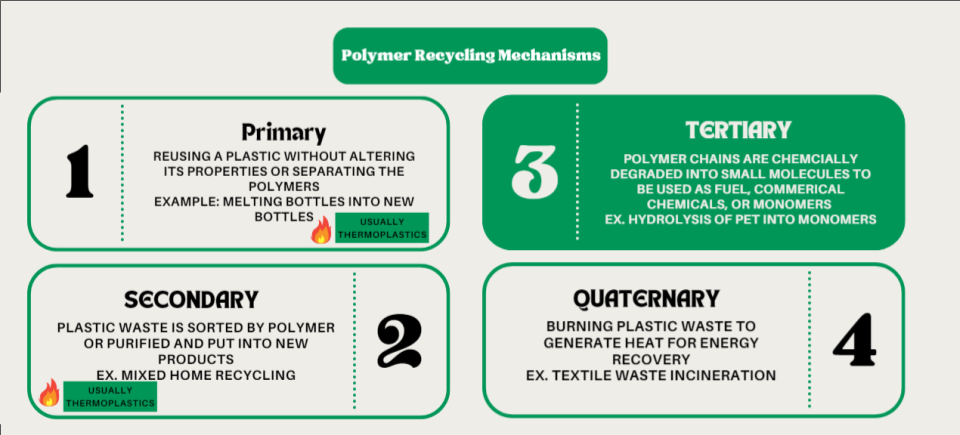
Figure 1. The four main types of polymer recycling. Information adapted from Ignatyev et al 20143.
Each recycling technique has advantages and disadvantages for any given polymer. Primary and secondary recycling suffer from purity issues as post-consumer waste may contain food residues or labels. Secondary recycling is especially challenging due to the need to sort plastics accurately. In addition, the heat used in melting the polymers may damage the chain structures and reduce their physical properties. Tertiary recycling often requires specialized catalysts, potentially concerning chemicals, and also suffers from purity concerns. Pyrolysis especially is energy intensive due to the need for high temperatures. Quaternary recycling is largely universal but generates problematic byproducts such as CO2, char, and other potentially dangerous gases.
This chapter focuses on chemical recycling because it is the most relevant to the polymer chains and produces a wide range of useful products for a more circular plastics industry. The various methods to depolymerize a material depending on the structure of the backbone, the types of chemicals produced, and their uses will be discussed. Finally, a research article on chemical recycling will be presented to introduce academic research on chemical recycling.
Polymer Specific Techniques
Unfortunately for chemical recycling enthusiasts, there are limited “magic bullet” techniques to depolymerize every commercially available plastic. The structure of the polymer plays a significant role in how it can be broken down. Polymers containing heteroatoms (atoms other than carbon) are often easier to break down than olefins, which are polymers that only contain carbon along the backbone.
Polyethylene Terephthalate (PET)
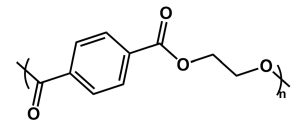 |
| Figure 2: The structure of the repeat unit of PET. |
Polyethylene terephthalate (structure seen in Figure 2) is one of the most common polymers used in hard plastics and textile fibers. It is a transparent thermoplastic, impermeable to gases, reasonably strong, and meltable at an achievable temperature. For those reasons, it is manufactured in high volumes and is commonly used in bottles and other packaging applications. Because it is a thermoplastic, it is easy to melt and reform into new products, but the high heat used to do so significantly reduces the mechanical properties of the polymer, reducing the material’s value4. The strain at break decreases from 35% to 0.7% after five thermal recycling cycles5. The limited capacity to recycle PET through primary and secondary recycling makes it an excellent candidate for tertiary recycling (depolymerization).
Looking closely at the backbone of PET relative to depolymerization, the ester bond immediately jumps out as the likely location for the simplest reaction. Hydrolysis, or adding a water molecule, is one of the most common methods to break down PET into its monomers (terephthalic acid and ethylene glycol). This reaction can be done under 3 conditions4:
- Acidic (pH < 7): most commonly using sulfuric acid (see Reaction 1 below)
- Basic (pH > 7): aqueous 4–20 wt% NaOH
- Neutral (pH ≈ 7): water, heat, and catalysts
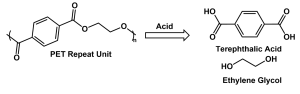
Reaction 1: The depolymerization of PET with acid into terephthalic acid and ethylene glycol.
All three hydrolysis conditions can depolymerize PET into its monomers (or their salts) as shown in Reaction 1 for acid hydrolysis, but have challenges. Acidic hydrolysis typically requires high concentrations of economically, industrially, and environmentally concerning acid and ultimately necessitate intensive separations. Basic hydrolysis takes longer than acid hydrolysis and requires heat which can further increase the costs and reduce the sustainable gains of recycling. Finally, neutral hydrolysis also requires high processing temperatures, higher pressures, and a higher ratio of water to polymer.4
Other chemical reactions can also break down PET at the ester linkage. These include methanolysis, glycolysis, aminolysis, and ammonolysis which are further described below6:
- Methanolysis (or other alcoholysis): the addition of methanol or another alcohol to PET resulting in dimethyl terephthalate (DMT) and ethylene glycol
- 180 – 280 ℃ at 2 – 4 MPa
- Glycolysis: the addition of ethylene glycol (or other glycols) resulting in one PET repeat unit with ethylene glycol units on both ends also called bis(2-Hydroxyethyl) terephthalate and ethylene glycol
- 160 – 260 ℃ in various solvents with many catalyst types
- Aminolysis: the addition of a primary amine resulting in a diamide based on terephthalic acid with hydrocarbon groups on either side and ethylene glycol
- 20 – 100 ℃, aqueous medium
- Ammonolysis: the addition of ammonia resulting in a diamide with CONH2 groups on either side along with ethylene glycol
- 70 – 180 ℃ under high pressure, ethylene glycol medium
Again, each method has its advantages and challenges. Alcoholysis with methanol is especially valuable because it generates DMT, which is often used to manufacture PET at industrial scales, but it can be difficult to separate the variety of products generated. However, the reaction results can be tuned by modifying the conditions such as temperature and pressure, making alcoholysis a diverse and useful tool. However, it generally requires high temperatures and catalysts, which can be ruined by the presence of water. Looking to the future, supercritical alcohols are being investigated because of their apparent success. Glycolysis has the historical advantage of significant research and may be one of the oldest methods used to degrade PET chemically, but it often necessitates catalysts like many methods6. In general, chemolysis of PET requires high temperatures, necessitates product separation, and often catalysts, which can only be used so many times. However, they generate industrially valuable chemicals that can be used to make new plastics without losing physical properties.
Polyethylene (PE)
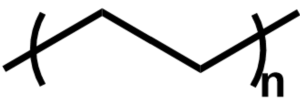 |
| Figure 3: The structure of the repeat unit of PE. |
Polyethylene (PE) is a common plastic in many industries including packaging and manufacturing. Comprised of a long unsubstituted alkane chain as seen in Figure 3, it is relatively simple and inexpensive to manufacture. It comes in a variety of densities and chain lengths thus dubbed low-density PE (LDPE), which is highly branched; high-density PE (HDPE), which has minimal branching; and ultra-high molecular weight PE (UHMWPE) which has a high degree of polymerization. Each type affects the crystallinity and therefore other properties such as opacity and strength. PE is a thermoplastic and can be mechanically recycled by melting, but this process suffers contamination and degradation challenges.
As an olefin polymer, which is a polymer that only contains carbon along the backbone, PE is recycled in a very different manner from PET, which has a more easily broken ester linkage. For that reason, PE is often broken down using pyrolysis, but this produces a wide and difficult-to-control range of products making it less exact than chemolysis. In addition, it requires temperatures over 500 ℃ and is therefore energy intensive7. An alternative method is breaking the C-C bonds using various catalysts. First, the polymer is partially unsaturated using various reactions such as bromination and a subsequent base and various iridium/ruthenium catalysts. The partially unsaturated structure can then be functionalized for simpler depolymerization and re-polymerization7. Similarly, long-chain aliphatic polyesters can mimic PE’s structure of long alkyl chains but have the advantage of breakpoints for hydrolysis or other chemolytic recycling processes8.
Polypropylene (PP)
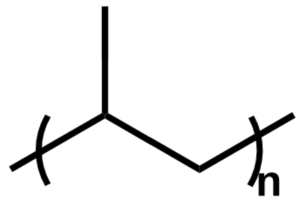 |
| Figure 4: The structure of the repeat unit of PP. |
Like PE, polypropylene (PP) is an olefin polymer and is comprised of a long alkyl chain. PP has the same backbone as PE except for a methyl group on every other carbon as seen in Figure 4. It is also commonly used in packaging and other industrial applications because of its low cost and favorable properties. Its structure can be isotactic if all the methyl groups face the same direction, syndiotactic if they face alternating directions, or atactic if their arrangement is random. The tacticity of the polymer depends on how it was synthesized and affects some of the polymer’s physical properties. It is also commonly pyrolyzed to generate oil, but this process is nonspecific and results in low monomer yields. However, it is difficult to break the C-C bonds along the backbone, so PP is generally not chemically recycled in the same way that polyester or other non-olefin polymers are.
Starch
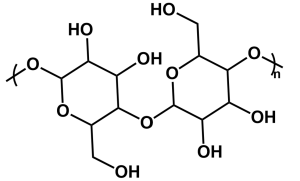 |
| Figure 5: The structure of a starch dimer. |
Given the current fears of waste accumulation and synthetic origins, many products and companies are turning towards bio-based polymers such as starch (Figure 5). Although it does not resemble petroleum-based plastics, starch is a valuable polymer once broken down into smaller units. Unlike olefin polymers, it has an ester linkage between repeat units which is the site for reaction. There are multiple mechanisms through which starch and other polysaccharides are degraded. These include:
- Acid-catalyzed hydrolysis
- Enzymatic hydrolysis
- Ionic liquid dissolution
Both acid and enzyme-catalyzed hydrolysis are similar to PET hydrolysis in that they attack the site of the heteroatoms. However, the ester bond is the site of this depolymerization. Unlike the other two methods, ionic liquid dissolution relies not on breaking the ester bond directly but rather on interrupting the hydrogen bonds between the chains9 so that water can better contact the ester linkages which are then broken. Unlike the oil-like products of pyrolysis and the synthetic monomers from PET, PE, and PP recycling, starch breaks down into monosaccharide or oligosaccharide units with many uses, largely in generating biofuels through fermentation.
General Chemical Recycling Techniques
Pyrolysis
The first major way to depolymerize and chemically break down plastic is through pyrolysis, heating the polymer at extremely high temperatures to break the bonds. Importantly, there is no oxygen in the pyrolysis reactor10, preventing the material from combusting which would otherwise ruin it. For PET, this reaction can occur at 450 ℃ and gives a variety of products including terephthalic acid, ethylene glycol, and short-chain polymers called oligomers. Both liquid and gas are generated and can be used similarly to oil as a synthetic precursor or fuel. Unfortunately, pyrolysis generates problematic by-products similar to quaternary recycling like CO2 and CO for PET11.
Because pyrolysis of plastics generally necessitates high temperatures, especially for olefins due to breaking carbon-carbon bonds. Zeolites, alkali metals and alkali earth metal hydrated aluminosilicates, can be used in conjunction with pyrolysis. Specifically, the brønsted acid sites assist in breaking down the polymers in more specific ways. Unfortunately, these catalysts can be quickly deactivated.12
Dissolution
As the name suggests, dissolution is the process of dissolving the polymer in a solvent. It is subsequently precipitated using a non-solvent to resolidify the polymer13. In doing so, the polymer can be separated from impurities to give a more pure recycled product. Unlike other chemical recycling techniques, the polymer chain remains intact throughout the process but is chemically separated from other components. Aside from unknown or unintentional impurities like food waste, plastics can be separated from other polymers in blended materials, enabling mixed plastic waste to become purified feedstocks which are otherwise difficult to reprocess, especially in post-consumer waste where the various compositions are unknown.
PET has been demonstrated to dissolve in N-Methyl-2-pyrrolidone and be re-precipitated in n-octane, but the researchers encountered challenges of high viscosity and low polymer concentrations (0.2 kg/L of solvent) along with high temperatures at 165 ℃. The resultant polymer was significantly less crystalline than virgin material but further recycling did not significantly affect the material’s properties14. Importantly, the researchers were able to recycle over 98% of the solvents used, significantly boosting this method’s sustainability.
Similar strategies have been demonstrated for recycling olefin polymers like PE and PP. Researchers were able to dissolve PP in xylene at 135 ℃ and precipitate it in acetone. Again, the solubility of the polymer is limited and was 0.15 kg/L of solvent. Interestingly, the crystallinity of the recycled material was higher than that of virgin material13. Researchers recently used α-Pinene, a component of many plant oils, to selectively dissolve HDPE with an ethanol antisolvent and recover 87% of the polymer15. However, α-Pinene is expensive and the researchers lost around 20% of the solvent throughout the process so it will likely need to be optimized before widespread industrial use.
Article Summary
Degradation of plastic wastes to commercial chemicals and monomers under visible light
Given the importance of sustainable waste management and the increased consumer awareness of plastic waste, many researchers are targeting both broad and specific chemical recycling knowledge. Meng and colleagues (2023) researched the use of visible light-sensitive catalysts to break down common plastic materials in an article titled, “Degradation of plastic wastes to commercial chemicals and monomers under visible light”16.
Unlike traditional polymer chemolysis for ester and ether polymers, the researchers used a uranium-based catalyst along with a blue LED light to break down 9 different types of plastic including polystyrene (PS), styrene acrylonitrile (SAN), PET, polybutylene terephthalate (PBT), and polycarbonate (PC). The uranyl catalyst was based on UO2 and can remove protons such as from a C-H system and can enable an oxygen transfer to cleave oxygen-containing bonds through hydrolysis. It can also participate in electron transfer with aromatic systems. All three possibilities make these catalysts an option to depolymerize multiple common polymers.
To degrade the plastics, the researchers treated the polymers with 5% catalyst for 72 hours under a blue LED light at 490 nm under either an oxygen or nitrogen atmosphere. Interestingly, the reactions were performed at room temperature in contrast to many of the techniques previously described in this chapter. Depending on the polymer, HCL, trichloroacetic acid, or ZnBr2 were added. They also tested the experiment with post-consumer PET bottle waste in a more continuous setup to produce terephthalic acid. Ultimately, the researchers determined that the blue light was significant in breaking the polymer apart, as evidenced by viscosity measurements seen in Figure 6.
 |
| Figure 6: The viscosity of samples treated with uranyl catalyst without blue light (blue) and with blue light irradiation (orange).16 |
See What You Know!
Take this short quiz to see what you learned…
References