15 Differential Scanning Calorimetry (DSC) in Characterizing Polymer Crystallinity and Thermal Properties
mnazem
Learning Objectives
Upon Completion of this chapter students will be able to:
- Explain the role of DSC in thermal analysis
- Evaluate the impact of thermal properties on the polymer performance
- Define polymer crystallinity and its measurement
- Apply DSC for material identification
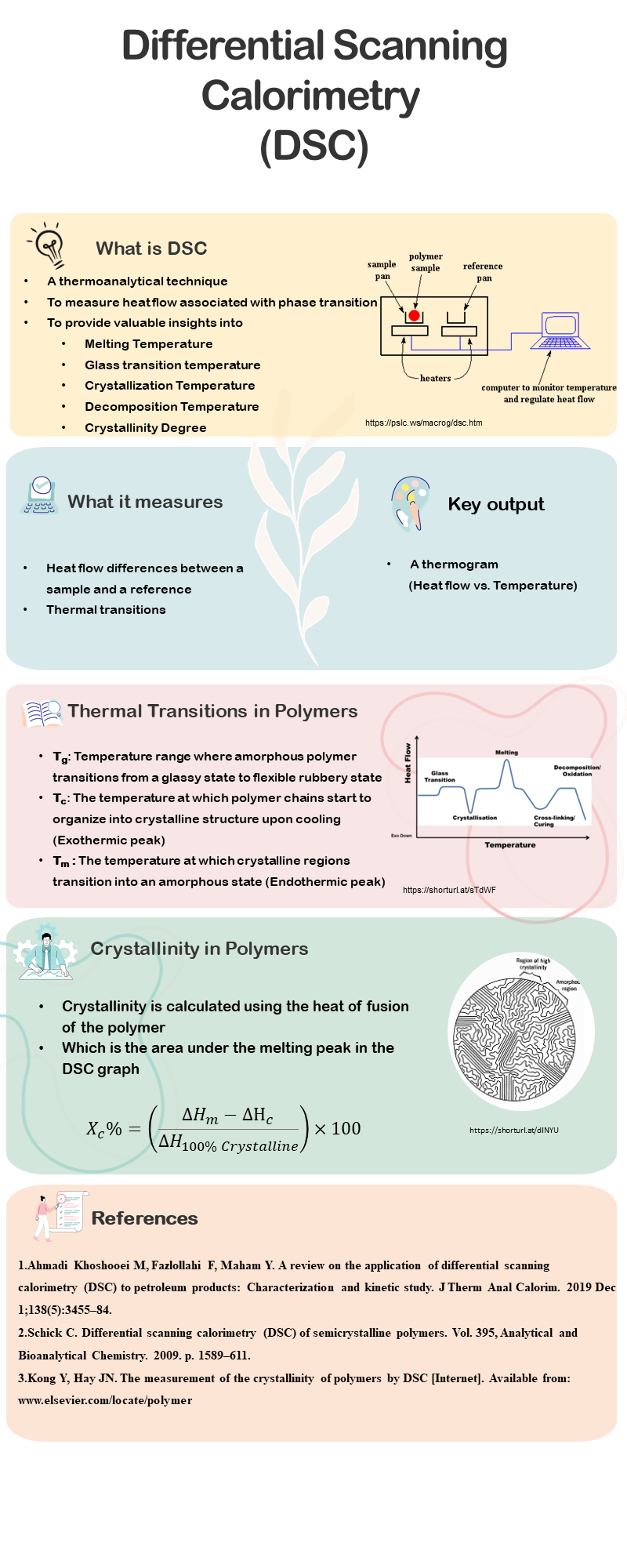
Introduction to Differential scanning Calorimetry
Calorimetry in polymer science originated in the first half of the 20th century but became a standard technique with the introduction of differential scanning calorimeters (DSC) in the early 1960s. DSC revolutionized calorimetry by offering a broad dynamic range of heating and cooling rates, including isothermal and temperature-modulated modes. Modern DSC systems achieve scanning rates spanning 12 orders of magnitude, from as low as 1 μK/s to rates exceeding 1 MK/s for both heating and cooling, providing unparalleled versatility (Schick, 2009)
This extensive range is particularly beneficial for studying semicrystalline polymers, which often exist far from equilibrium and exhibit time-dependent phase transitions. Despite its utility, challenges in polymer calorimetry persist, such as accurately determining baseline heat capacity, resolving issues in crystallinity measurements, and addressing the occurrence of multiple melting peaks. These challenges emphasize the need for ongoing refinement in DSC methodologies (Schick, 2009).
Differential Scanning Calorimetry is a widely used thermal analysis technique that examines material properties by analyzing energy changes during heating or cooling. It provides critical insights into phase transitions, thermal stability, and thermodynamic properties such as enthalpy, entropy, and heat capacity, facilitating a deeper understanding of a material’s structure and composition (Ahmadi Khoshooei et al., 2019).
DSC finds applications across diverse fields, including polymer science, pharmaceuticals, and food technology. It is particularly effective in detecting phase transitions such as melting, crystallization, and glass transitions, while quantifying the associated energy changes. These features make DSC an essential tool for material characterization and quality control (Ahmadi Khoshooei et al., 2019; Schick, 2009).
Furthermore, combining DSC with complementary analytical techniques enhances its utility by enabling validation of findings and providing a more comprehensive understanding of material properties (Ahmadi Khoshooei et al., 2019).
Modes of Operation in DSC
Differential scanning calorimeters usually have two positions: one for the sample being tested and the other for a reference, which is typically an empty crucible or one filled with an inert material (Schick, 2009).
DSC systems can operate in two main configurations: two-dimensional and three-dimensional measurement systems(Schick, 2009).
Two-Dimensional Systems
In most heat flow DSCs and ultra-fast scanning calorimeters, the sample exchanges heat with the surrounding oven, bypassing the heat flow sensor. Up to 50% of the total heat flow may not be measured by the sensor. These losses can be accounted for during calibration, using methods like Tzero technology, but they still limit the accuracy of the measurements(Schick, 2009).
Three-Dimensional Systems
Three-dimensional systems, such as Tian-Calvet calorimeters, solve this issue by ensuring nearly all heat exchange occurs through the sensing element, achieving up to 94% accuracy. These systems can measure heat capacity and latent heat with about 1% accuracy but have a drawback: their slow response time, making them less suitable for studying semicrystalline polymers(Schick, 2009).
Quasi Three-Dimensional Systems
A modified approach, developed by Watson and O’Neill, uses power-compensating DSCs where the sample and reference are placed in separate, actively controlled ovens. This design improves accuracy to about 0.5% under ideal conditions and 2% in routine use. In temperature-modulated modes, the accuracy can improve to better than 1%(Schick, 2009).

Figure 1 – Various types of differential scanning calorimeters (DSC) include ((a) two-dimensional plate designs, (b) three-dimensional cylindrical models such as the Tian-Calvet type, and (c) three-dimensional systems with power compensation) (Schick, 2009).
Each mode offers a trade-off between accuracy, speed, and application, depending on the material and experimental conditions.
Applications of DSC in Polymer Research
DSC plays a pivotal role in polymer research, particularly in analyzing key thermal transitions such as melting, crystallization, and glass transition temperatures. It measures critical energy changes, including latent heat and variations in heat capacity, offering insights into a polymer’s structure, composition, and performance. These measurements are vital for understanding how polymers behave under different thermal conditions, which informs material design and optimization (Schick, 2009).
In addition to its role in materials engineering and quality control, DSC is extensively used to investigate thermal stability and phase behavior in polymer systems. The technique helps scientists and manufacturers develop and enhance polymers for diverse applications, making it a cornerstone in modern polymer science (Schick, 2009).

Figure 2 – Different applications of DSC (Drzeżdżon et al., 2019)
Thermal Properties of Polymers Analyzed by DSC
From thermodynamics, understanding a material’s heat capacity behavior from absolute zero (0 Kelvin) to the temperature of interest provides valuable insights into several key material properties(Schick, 2009):

Heat Capacity
Heat capacity is a crucial thermal property for understanding material behavior across a wide temperature range, but challenges arise at higher temperatures or in low thermal conductivity materials like polymers, where time-dependent variations increase measurement uncertainties. Differential Scanning Calorimetry (DSC) effectively addresses these challenges, providing reliable and efficient heat capacity measurements and enabling the study of transition kinetics over a broad dynamic range. Its simplicity and versatility make DSC an indispensable tool in polymer science, with its foundation rooted in the relationship between heat flow and material transitions(Schick, 2009).
![]()
or, in differential form, assuming time independent sample mass and specific heat capacity(Schick, 2009):

Key Thermal Parameters
Differential Scanning Calorimetry (DSC) technique is used to study phase transitions and thermodynamic properties of materials. It provides valuable information about key thermal parameters such as glass transition temperature (Tg), melting temperature (Tm), crystallization temperature (Tc), and decomposition temperature (Td) (Leyva-Porras et al., 2020).
Glass Transition Temperature (Tg)
Tg is a second-order transition observed in polymeric systems, characterized by changes in molecular mobility and relaxation time in amorphous solids. Unlike first-order transitions, such as melting, which involve abrupt changes in specific volume, Tg represents a continuous change in the slope of the specific volume-temperature curve. This transition occurs due to variations in temperature, pressure, or humidity and marks the shift between glassy and rubbery states. Tg is considered a state transition rather than a phase transition due to the non-equilibrium nature of the glassy state and its occurrence over a temperature range. At Tg, molecules gain enough free volume to move relative to one another, enabling the material to transition from rigid to flexible (Leyva-Porras et al., 2020).
Melting Temperature (Tm)
Tm represents the temperature at which a crystalline material melts. It appears as an endothermic peak in the DSC curve, indicating the absorption of heat during the melting process (Leyva-Porras et al., 2020).

Figure 3 – Diagram illustrating first- and second-order transitions: (a) Melting transition in a crystalline solid, and (b) Glass transition in an amorphous solid (Leyva-Porras et al., 2020).
Crystallization Temperature (Tc)
Tc is the temperature at which a material crystallizes from its amorphous or liquid state. It is observed as an exothermic peak in the DSC thermogram, signifying the release of heat during crystallization (Leyva-Porras et al., 2020).
Decomposition Temperature (Td)
Td is the temperature at which a material begins to thermally decompose. It is typically characterized by a significant endothermic peak or a sudden change in the baseline of the DSC curve (Leyva-Porras et al., 2020).

Figure 4 – Illustration of thermal transitions in semicrystalline materials as observed in a differential scanning calorimetry (DSC) thermogram (Leyva-Porras et al., 2020).
Degree of Crystallinity in Polymers
The degree of crystallinity is a critical property of polymers, influencing mechanical properties like yield stress, elasticity, and impact resistance. For example, amorphous PET has poor dimensional stability and gas permeability, making it less useful compared to crystalline PET, which offers superior strength, stability, and chemical resistance. Crystallinity plays a vital role in applications such as fibers and beverage containers due to its impact on strength and gas permeability (Kong & Hay, n.d.; Leyva-Porras et al., 2020).
Crystallinity is temperature-dependent, making it important to conduct measurements at consistent temperatures to ensure reliable comparisons of material properties (Kong & Hay, n.d.).
Several techniques are used to determine polymer crystallinity, including wide-angle X-ray diffraction (WAXD), density measurements, and differential scanning calorimetry (DSC). DSC is the most widely used method due to its ability to measure heat of fusion. However, the approach often relies on drawing arbitrary baselines and assumes no recrystallization or remelting during the process, leading to discrepancies with other methods (Kong & Hay, n.d.).
DSC calculates the degree of crystallinity by integrating the melting peak and comparing the measured heat of fusion with the theoretical heat of fusion of a 100% crystalline material. The formula used is (Kong & Hay, n.d.):
![]()
where is the heat of fusion at the melting temperature, and is the heat of fusion for a fully crystalline polymer at equilibrium melting temperature. However, this calculation does not account for specific heat changes or other temperature-dependent factors, introducing inaccuracies (Kong & Hay, n.d.).
In case sample crystallization occurs during heating, an exothermic peak is observed during cold crystallization, followed by an endothermic peak during melting at distinct temperature regions. The degree of crystallinity (XcXc) is then calculated using the following equation (Kong & Hay, n.d.):

This equation accounts for both crystallization and melting to provide a more accurate determination of crystallinity (Kong & Hay, n.d.).
ATHAS Database
The ATHAS (Advanced Thermal Analysis System) database is a comprehensive resource providing standardized data for fully crystalline polymers, such as the heat of fusion, aiding in accurate calculations of crystallinity and phase transitions (Schick, 2009).
Challenges in DSC for Polymer Analysis
Differential Scanning Calorimetry (DSC) is a widely used technique to calculate the heat of fusion and determine the degree of crystallinity in semicrystalline materials. However, this process can be complex for polymers due to several factors (Schick, 2009):
- Broad melting ranges and multiple melting peaks complicate the integration of the melting curve.
- The presence of a rigid amorphous fraction, along with crystalline and liquid amorphous phases, requires careful consideration during thermal analysis.
- The heat of fusion for fully crystalline materials is typically referenced at equilibrium melting temperatures, which differ from observed values, necessitating temperature-dependent corrections.
- Variations in heat capacity between liquid and crystalline phases result in non-parallel enthalpy curves, adding to the complexity of analysis.
These challenges emphasize the importance of precise baseline construction and the need for advanced methods to improve the accuracy of crystallinity determination.
(Schick, 2009).
Peer Reviewed Article
Thermal properties and decomposition products of modified cotton fibers by TGA, DSC, and Py–GC/MS
This article focuses on characterizing modified cotton fibers through various thermal analysis techniques, including Differential Scanning Calorimetry (DSC). The study investigates the effects of two chemical treatments—silanization and a novel sulphation-phosphorylation approach—on cotton fibers. The research highlights the significant changes in thermal stability, decomposition pathways, and pyrolysis products due to these modifications (Isola et al., 2024).
Key findings include (Isola et al., 2024):
- Silanization improves thermal stability and hydrophobicity while favoring cellulose depolymerization over dehydration.
- The sulphation-phosphorylation method enhances flame retardancy by promoting char formation and reducing flammable decomposition products.
- DSC analysis reveals distinct thermal transitions for the modified fibers, reflecting the impact of the chemical treatments on fiber properties.
This study is highly relevant for industrial applications requiring flame-retardant and thermally stable fibers, such as textiles and composites (Isola et al., 2024).
The chapter provides a theoretical foundation for understanding the principles and methodologies of DSC, which are directly applied in the article to assess thermal transitions, such as dehydration and decomposition, in modified fibers. By exploring DSC’s role in determining crystallinity and thermal stability, the chapter connects to the article’s use of DSC to evaluate the impacts of modifications on cotton fibers’ thermal stability and flame-retardant properties. Additionally, the challenges outlined in the chapter, such as baseline construction and the complexity of analyzing semicrystalline materials, are mirrored in the article’s detailed approach to thermal analysis.
Conclusion
Differential Scanning Calorimetry (DSC) is a highly useful tool in polymer science for characterizing material properties, including thermal transitions, crystallinity, and heat capacity. Its versatility and precision enable researchers to analyze complex thermal behaviors and gain insights into the structure and performance of polymers. While DSC provides significant advantages, such as a broad dynamic range and high sensitivity, challenges remain in areas like baseline determination and interpreting transitions in semicrystalline materials. Ongoing advancements in DSC techniques continue to enhance its applicability, solidifying its role in both fundamental research and industrial applications.
References
Ahmadi Khoshooei, M., Fazlollahi, F. & Maham, Y. (2019). A review on the application of differential scanning calorimetry (DSC) to petroleum products: Characterization and kinetic study. Journal of Thermal Analysis and Calorimetry, 138(5), 3455–3484. https://doi.org/10.1007/s10973-019-08244-2
Drzeżdżon, J., Jacewicz, D., Sielicka, A. & Chmurzyński, L. (2019). Characterization of polymers based on differential scanning calorimetry based techniques. In TrAC – Trends in Analytical Chemistry (Vol. 110, pp. 51–56). Elsevier B.V. https://doi.org/10.1016/j.trac.2018.10.037
Isola, M., Colucci, G., Diana, A., Sin, A., Tonani, A. & Maurino, V. (2024). Thermal properties and decomposition products of modified cotton fibers by TGA, DSC, and Py–GC/MS. Polymer Degradation and Stability, 228. https://doi.org/10.1016/j.polymdegradstab.2024.110937
Kong, Y. & Hay, J. N. (n.d.). The measurement of the crystallinity of polymers by DSC. www.elsevier.com/locate/polymer
Leyva-Porras, C., Cruz-Alcantar, P., Espinosa-Solís, V., Martínez-Guerra, E., Piñón-Balderrama, C. I., Martínez, I. C. & Saavedra-Leos, M. Z. (2020). Application of differential scanning calorimetry (DSC) and modulated differential scanning calorimetry (MDSC) in food and drug industries. In Polymers (Vol. 12, Issue 1). MDPI AG. https://doi.org/10.3390/polym12010005
Schick, C. (2009). Differential scanning calorimetry (DSC) of semicrystalline polymers. In Analytical and Bioanalytical Chemistry (Vol. 395, Issue 6, pp. 1589–1611). https://doi.org/10.1007/s00216-009-3169-y
