40 Hydrogel Polymers: Structure-Property Relationships in Biomedical Scaffolds
Direselgn Semanie
Learning Objectives
By the end of this chapter, readers will be able to:
- Describe the fundamental structural characteristics that define hydrogel polymers.
- Explain how cross-linking density and network architecture influence key hydrogel properties such as swelling behavior, mechanical strength, and degradation.
- Analyze the relationship between hydrogel material properties (e.g., stiffness, porosity, bioactivity) and their performance in biomedical scaffold applications.
- Evaluate recent advances in smart or responsive hydrogels (e.g., pH-, temperature-, or enzyme-sensitive) for targeted therapeutic and tissue engineering applications.
- Critically discuss current research challenges and future directions in the design of hydrogel-based scaffolds.
Introduction
Hydrogels are three-dimensional, cross-linked polymeric networks capable of absorbing and retaining large amounts of water or biological fluids while maintaining their structural integrity. Their unique combination of soft, tissue-like mechanical properties, high water content, and biocompatibility makes them exceptionally suitable for biomedical applications, particularly as scaffolds for tissue engineering and regenerative medicine. The performance of hydrogels in these roles is intimately tied to the relationship between their molecular structure, physical properties, and functional behavior, a core theme of this chapter. Understanding these structure-property relationships is essential for designing next-generation hydrogel systems that can mimic the native extracellular matrix, support cell growth, and respond to physiological cues.
The history of hydrogels dates back to the 1960s with the work of Wichterle and Lím, who developed poly(2-hydroxyethyl methacrylate) (pHEMA), the first synthetic polymer used in soft contact lenses. Since then, the field has exploded, moving beyond simple materials to sophisticated smart hydrogels that can respond dynamically to external stimuli like pH, temperature, light, or the concentration of specific molecules.
The basic structure of a hydrogel consists of a polymer backbone that forms a network, often through physical associations (such as hydrogen bonding, hydrophobic interactions, or chain entanglement) or chemical covalent bonds (chemical cross-linking). It is the density of these cross-links that primarily dictates the network’s structural integrity, mechanical strength, and, crucially, the amount of water it can absorb. For instance, a higher cross-link density generally results in a stiffer hydrogel with lower swelling capacity, directly illustrating a key structure-property relationship. The chemical nature of the polymer chains, whether they are non-ionic, ionic (anionic or cationic), or amphiphilic, determines the specific interactions with water and solutes, influencing properties like pH sensitivity and drug loading capacity.
Structural Determinants of Hydrogel Networks
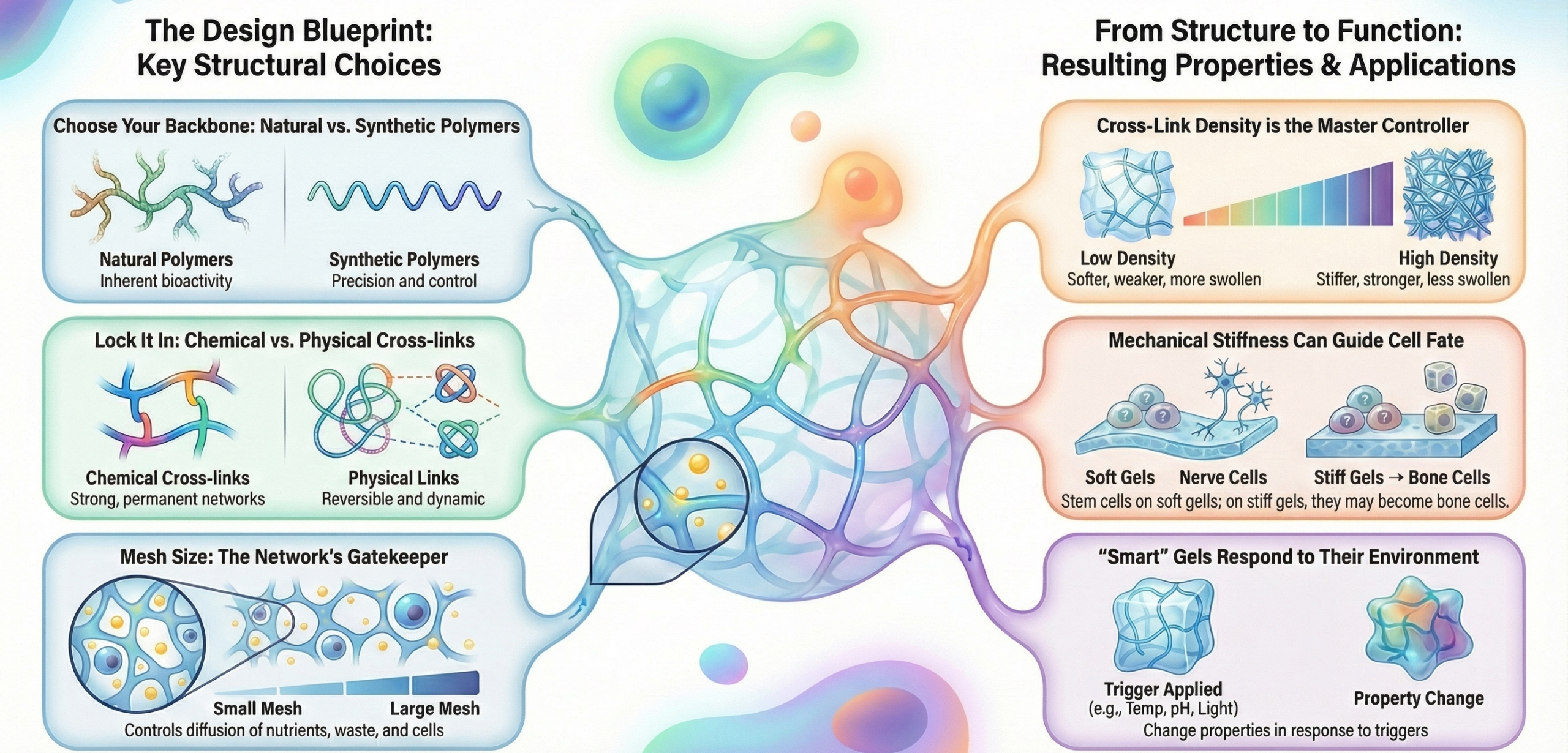
The blueprint of a hydrogel’s behavior is established at the molecular and network levels during its formation. Three primary structural factors dictate its destiny: the polymer backbone chemistry, the nature and density of cross-links, and the resulting network architecture.
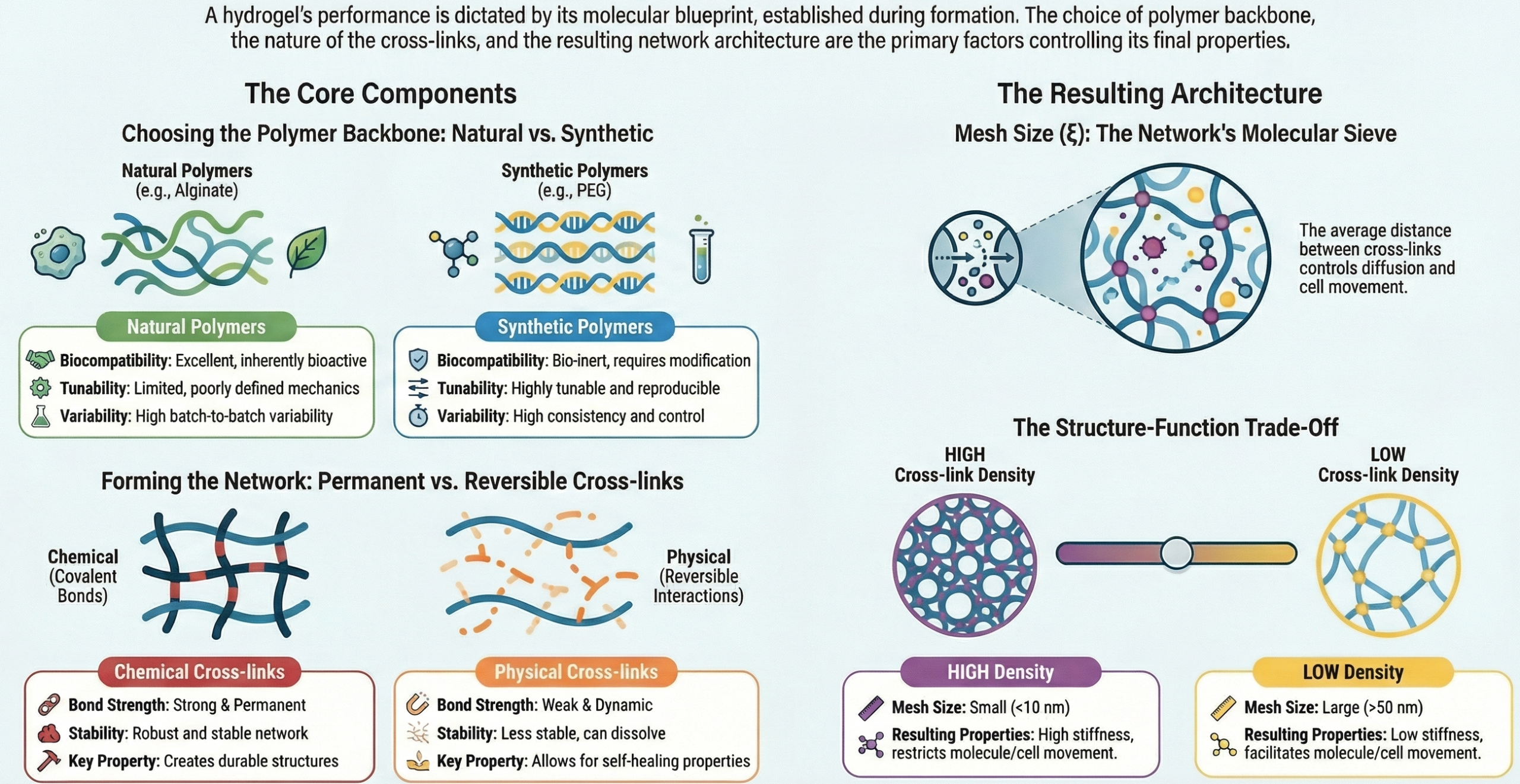
Polymer Backbone and Chemical Composition
The choice of polymer(s) is the first and most fundamental decision. Hydrogels can be derived from natural, synthetic, or hybrid polymers, each conferring distinct advantages and challenges.
Natural Polymers (e.g., Collagen, Fibrin, Hyaluronic Acid, Alginate, Chitosan)
These materials, often components of the native extracellular matrix (ECM), inherently possess excellent biocompatibility and bioactivity. They frequently contain cell-adhesion motifs (like the RGD sequence in collagen) and can be degraded by naturally occurring enzymes (e.g., matrix metalloproteinases). However, they can suffer from batch-to-batch variability, potential immunogenicity, and often have relatively weak and poorly defined mechanical properties. Alginate, for example, forms gentle ionically cross-linked gels ideal for cell encapsulation but lacks mammalian cell adhesion sites unless modified.
Synthetic Polymers (e.g., Poly(ethylene glycol) [PEG], Poly(vinyl alcohol) [PVA], Poly(hydroxyethyl methacrylate) [PHEMA])
These offer unparalleled control over consistency, molecular weight, and structure. They are highly tunable and reproducible. PEG is the “gold standard” for its hydrophilicity and resistance to protein fouling, creating a biologically “blank slate.” However, synthetic polymers are typically bio-inert, lacking inherent cell signals and enzymatic degradation sites. Therefore, they require deliberate functionalization to become bioactive.
Hybrid/Semi-Synthetic Polymers
This powerful approach seeks the best of both worlds. For instance, PEG can be copolymerized with degradable peptides or grafted with hyaluronic acid. This creates materials with the defined mechanical properties of a synthetic system and the specific biological interactions of a natural one.
Cross-Linking
Cross-links are the junctions that connect polymer chains into a cohesive, insoluble network. The cross-linking density (ρx), defined as the number of cross-links per unit volume, is arguably the single most important parameter controlling hydrogel properties.
- Chemical (Permanent) Cross-Linking: Involves the formation of covalent bonds between polymer chains. Methods include:
- Chain-Growth Polymerization: Using multi-functional monomers (e.g., PEG diacrylate) and a free radical initiator, often activated by light (photopolymerization). This allows for spatial and temporal control, enabling 3D printing or injection followed by in-situ gelation.
- Step-Growth Reactions: Click chemistry (e.g., azide-alkyne cycloaddition), Schiff base formation, or reactions between complementary groups (e.g., NHS esters with amines) offer high efficiency and specificity.
- High-Energy Radiation: Using gamma or electron beams to generate radicals on polymer chains (e.g., PVA), which then recombine to form cross-links. Chemical cross-links create stable, robust networks but may require cytotoxic initiators or produce non-degradable bonds.
- Physical (Reversible) Cross-Linking: Involves non-covalent, dynamic interactions that can often reform after breakage, imparting self-healing and shear-thinning properties.
- Ionic Interactions:Divalent cations (Ca²⁺, Ba²⁺) cross-linking alginate chains.
- Crystallite Formation: In PVA hydrogels where freeze-thaw cycles create crystalline domains acting as physical cross-links.
- Hydrogen Bonding & Hydrophobic Interactions: Seen in some block copolymers or self-assembling peptides.
- Supramolecular Chemistry: Host-guest interactions (e.g., cyclodextrin and adamantane) or metal-ligand coordination. Physically cross-linked gels are often gentler for cell encapsulation but can be less mechanically stable and may dissolve under specific ionic or thermal conditions.
Network Architecture and Mesh Size (ξ)
The arrangement of polymer chains and cross-links defines a porous network. The mesh size (ξ)is the average linear distance between two adjacent cross-links. It is a derived property, inversely related to cross-linking density ( for an ideal network). The mesh size acts as a molecular sieve, controlling the diffusion of nutrients, oxygen, waste products, and even cells through the hydrogel. A small mesh size (< 10 nm) restricts macromolecule diffusion but provides a dense, stiff microenvironment. A large mesh size (> 50 nm) facilitates mass transport and can allow for cell infiltration and vascularization but at the cost of reduced mechanical integrity.
Structure, Physical and Mechanical Properties
The structural determinants described above directly and quantitatively manifest in the hydrogel’s macroscopic behavior.
Swelling Behavior and Thermodynamics
A hydrogel’s defining characteristic is its capacity to absorb solvent, typically water, and undergo substantial swelling without dissolving. This process is governed by a balance of opposing thermodynamic contributions
- Driving Force (Mixing Entropy):When a dry polymer network is exposed to water, the mixing of solvent molecules and polymer chains results in a favorable increase in configurational entropy, denoted as . This entropic gain is the primary driver for solvent absorption.
- Opposing Force (Elastic Retraction):As the network imbibes solvent and expands, the polymer chains between cross-links are stretched. This deformation generates an elastic retractive force that resists further swelling, associated with an increase in the elastic free energy, ΔGel .
The gel reaches an equilibrium swelling state when the net change in Gibbs free energy for the swelling process is zero (ΔGswell=0). This condition is met when the chemical potential of the solvent inside the gel equals that of the pure solvent outside. The quantitative balance is often described by the Flory-Rehner theory, which relates the equilibrium swelling ratio to the network’s cross-link density and the polymer-solvent interaction parameter, χ.
A fundamental metric for quantifying this behavior is the swelling ratio, Q. It is typically defined as the mass of water taken up per unit mass of dry polymer:
Q=(mswollen-mdry)/mdry
where mswollen and mdry are the masses of the gel in the dry and equilibrium-swollen states, respectively. A high swelling ratio is essential for applications requiring high porosity and mechanical compliance, such as in contact lenses, drug delivery systems, and tissue engineering scaffolds.
- Structural Influence: A higher cross-linking density increases the elastic retractive force, limiting swelling (lower Q). More hydrophilic polymer chains increase the osmotic driving force, promoting swelling (higher Q). The mesh size increases with swelling. Swelling is critical for scaffold dimensions, nutrient diffusion, and can exert physical forces on encapsulated cells.
Mechanical Properties
The mechanical milieu is a powerful regulator of cell fate (a phenomenon known as mechanotransduction). Hydrogel stiffness is typically characterized by the elastic (or Young’s) modulus (E).
- Structural Influence:Modulus increases dramatically with increasing cross-linking density (E ~ ρx). The polymer backbone chemistry also plays a role; stiffer polymer chains (e.g., with aromatic groups) contribute to a higher modulus than flexible chains (e.g., PEG). For dynamic or viscoelastic hydrogels (most biological tissues are viscoelastic), properties like stress relaxation and energy dissipation are equally important and are tuned by incorporating reversible cross-links.
- Biological Impact:Stem cells differentiate down neurogenic lineages on soft substrates (~0.1-1 kPa), myogenic on intermediate stiffness (~8-17 kPa), and osteogenic on stiff substrates (>25 kPa). A scaffold for cartilage (a load-bearing tissue) requires a high modulus and toughness, while a scaffold for brain tissue requires a very soft, compliant gel.
Degradation Profile
A scaffold must often provide temporary support, degrading in sync with new tissue formation. Degradation can occur via:
- Hydrolysis:Cleavage of hydrolytically labile bonds in the backbone (e.g., esters, anhydrides) or cross-links. The rate depends on chemical structure, pH, and local hydrophobicity.
- Enzymatic Degradation:Incorporation of peptide sequences cleavable by cell-secreted enzymes (e.g., MMPs). This enables cell-demanded, localized remodeling.
- Structural Influence:Degradation rate is inversely related to cross-linking density. A denser network is more resistant to both chain cleavage and enzyme penetration. Degradation products must be non-toxic and readily cleared. As a hydrogel degrades, its swelling ratio and modulus change dynamically, which must be accounted for in design.
Functional Properties for Biomedical Scaffolds
Biocompatibility and Bioactivity
Biocompatibility extends beyond mere non-toxicity; it is the ability to support desired cellular functions.
- Cell Adhesion:Most synthetic and some natural hydrogels lack inherent cell adhesion sites. This is addressed by covalently coupling cell-adhesive peptides (e.g., RGD, IKVAV, YIGSR) to the polymer backbone. The density, spatial presentation, and affinity of these ligands profoundly influence cell attachment, spreading, and signaling.
- Bioactive Signal Presentation:Beyond adhesion, growth factors (e.g., VEGF for angiogenesis, BMP-2 for osteogenesis) can be physically entrapped or, more effectively, covalently tethered to the network. Tethering protects factors from rapid diffusion and degradation, presenting them in a stable, localized manner to cells. The hydrogel mesh size can be used to control the sustained release of soluble factors.
Mass Transport and Vascularization
A critical challenge for thick tissue constructs (>~200 µm) is the diffusion limit of oxygen. Cells in the center of a non-porous hydrogel will necrose.
- Structural Strategies: Creating larger, interconnected pore networks (increasing ξ) via techniques like porogen leaching, gas foaming, or cryogelation enhances convective flow. Alternatively, engineers design hydrogels with angiogenic potential by presenting VEGF and incorporating cell-degradable pathways to allow host blood vessels to infiltrate the scaffold post-implantation.
Smart and Responsive Hydrogels
A frontier in hydrogel design involves creating “smart” systems that change properties in response to specific biological or external triggers, enabling sophisticated spatial and temporal control.
- Stimuli-Responsive Swelling/Gelation:
- pH-Sensitive: Polymers with ionizable groups (e.g., poly(acrylic acid) swells at high pH; chitosan gels at neutral pH from an acidic solution) can be used for oral drug delivery or targeting inflamed (acidic) tissues.
- Thermoresponsive: Polymers like poly(N-isopropylacrylamide) (PNIPAAm) undergo a reversible sol-gel transition near body temperature, allowing for injectable, in-situ gelling systems.
- Photo-Responsive: Incorporation of molecules like spiropyran that change hydrophobicity upon light exposure can be used to dynamically alter stiffness or release payloads with high spatiotemporal precision.
- Dynamic and Adaptable Networks:
Incorporating reversible bonds (physical or dynamic covalent chemistry like Diels-Alder or disulfide exchange) creates hydrogels that can self-heal after damage or flow under shear stress (allowing injection) and then immediately reform. This is immensely valuable for minimally invasive delivery and for creating materials that can adapt and remodel with growing tissue.
Integration, Challenges, and Future Directions
Translating a designed hydrogel from the bench to the clinic requires integrating these concepts into a cohesive system. A scaffold for bone repair, for example, might combine a mechanically robust synthetic network (for load-bearing) with enzymatically degradable cross-links (for remodeling), RGD and BMP-2 peptides (for cell adhesion and differentiation), and a macroporous structure (for vascular invasion).
Challenges
- Mechanical Mismatch: Many hydrogels are too weak and brittle to match the toughness of native tissues like cartilage or muscle.
- Vascularization: Establishing a functional blood supply within large hydrogel constructs remains a major hurdle.
- Immune Response: Even “biocompatible” materials can elicit a foreign body response, leading to fibrotic encapsulation that isolates the implant.
- Manufacturing Complexity: Reproducibly fabricating scaffolds with hierarchical, biomimetic structures at multiple length scales is difficult.
Future Outlook
The field is moving towards fourth-generation hydrogels that are not just bioactive but also instructive and dynamic. This involves:
- Advanced Biofabrication:Using 3D bioprinting to deposit cell-laden hydrogels (bioinks) with precise spatial heterogeneity in chemistry, stiffness, and biofactors.
- Multi-Material and Graded Designs: Creating interfaces (e.g., bone-to-cartilage) with gradual property transitions.
- Feedback-Responsive Systems: Hydrogels that can sense and respond to local metabolic signals (e.g., glucose, reactive oxygen species) from the host tissue.
- Integration with Electronics: Developing conductive hydrogels (e.g., with polyaniline or graphene) for neural tissue engineering or biosensing.
References
-
Peppas, N. A., Hilt, J. Z., Khademhosseini, A., & Langer, R. (2006). Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Advanced Materials, 18(11), 1345–1360. https://advanced.onlinelibrary.wiley.com/doi/abs/10.1002/adma.200501612
-
Drury, J. L., & Mooney, D. J. (2003). Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials, 24(24), 4337–4351. https://pubmed.ncbi.nlm.nih.gov/12922147/
-
Hoffman, A. S. (2012). Hydrogels for biomedical applications. Advanced Drug Delivery Reviews, 64, 18–23. https://doi.org/10.1016/j.addr.2012.09.010
-
Seliktar, D. (2012). Designing cell-compatible hydrogels for biomedical applications. Science, 336(6085), 1124–1128. https://pubmed.ncbi.nlm.nih.gov/22654050/
Review (Non-planar additive manufacturing with hydrogels: a review of flow control and toolpath strategies)
https://www.nature.com/articles/s44431-025-00006-5
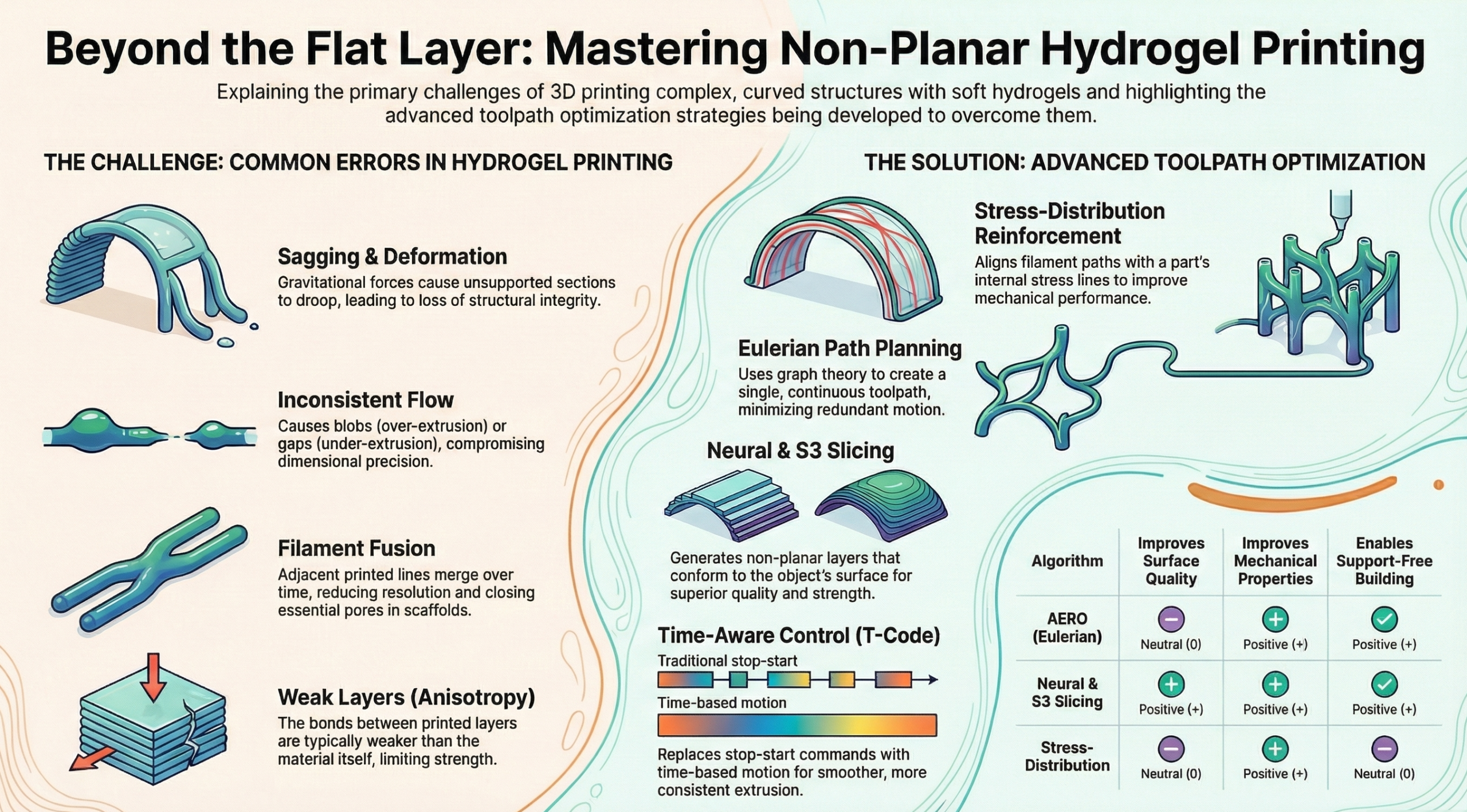
The paper “Non-planar additive manufacturing with hydrogels: a review of flow control and toolpath strategies” examines the advancements required to enable the fabrication of complex, freeform structures using hydrogels via extrusion-based additive manufacturing (AM). While hydrogels are highly promising for tissue engineering due to their high-water content, biocompatibility, and tunable rheology, their adoption in complex geometries is severely limited by issues like low stiffness, unstable flow, and poor structural fidelity in non-planar fabrication. The core objective of the review is to investigate how to achieve a stable and adaptable flow of hydrogel materials during multi-directional AM through the optimization of toolpath strategies. The review integrates key progress in extrusion control, slicing algorithms, and multi-axis systems to address these fabrication challenges.
The constraints of hydrogel polymers are central to the difficulties discussed. Hydrogels are defined as water-rich, three-dimensional polymer networks formed through chemical or physical cross-linking, enabling them to absorb and retain significant amounts of water, which is highly valued for biomedical applications. Mechanically, these materials typically exhibit low stiffness, generally below 100 kPa, and low toughness, limiting their capacity for standalone mechanical load bearing. While hydrogels benefit from viscoelastic and shear-thinning behavior, where viscosity temporarily decreases under shear rate for smooth extrusion and rapidly recovers for shape retention—maintaining this stable and controllable flow is challenging, particularly when the extrusion orientation changes in multi-directional AM. These inherent limitations necessitate specialized fabrication strategies to preserve the structural fidelity of the printed form.
To manage the soft, often slow-curing nature of hydrogels, the review highlights several material extrusion methods, many of which rely on external support. Techniques include Direct Ink Writing (DIW), which utilizes the material’s shear-thinning properties, and coaxial printing, which often uses a structurally supportive outer shell to contain a functional or bioactive core. Crucially, embedded 3D printing offers one of the most effective support-free strategies by depositing soft materials within a viscoelastic yield-stress fluid (a support bath), such as in the FRESH technique. This approach prevents material sinking or deformation, provided the yield stress of the bath ($\tau_y$) is sufficient to counteract the gravitational force ($\rho gh$) acting on the material. However, maintaining flow consistency remains difficult, leading to common hydrogel errors like sagging, over- or under-extrusion in corners, and filament fusion in porous architectures.
A significant portion of the paper focuses on advanced toolpath optimization to enable support-free, non-planar printing. Conventional layer-by-layer slicing is inadequate for complex geometries, leading to the necessity of Multi-axis AM (MAAM), which uses additional rotational degrees of freedom to deposit material along non-planar paths. The review details several advanced slicing algorithms, including Eulerian methods, which generate continuous, support-free paths by minimizing singularities in lattice structures. Furthermore, stress-aware slicing, such as Neural Slicing or heatmap-based optimization, is employed to align deposition paths with anticipated stress directions or isotherms, respectively, thereby improving the mechanical performance and surface finish of the printed structure. To enhance flow control during these complex motions, time-aware strategies like the T-code algorithm have been introduced, which redefine motion using synchronized velocity and acceleration profiles to minimize mechanical jerk and maintain uniform material deposition.
The review concludes that achieving successful non-planar AM of hydrogels requires unifying material properties, tool motion, and extrusion parameters into an adaptive toolpath planning framework. The advancements reviewed hold significant translational potential for biomedical applications, demonstrated by the creation of skin tissue scaffolds, vascular structures (often via coaxial printing and sacrificial supports), and soft robots. Ultimately, future research must focus on adaptive algorithms for dynamic flow regulation, real-time in situ correction, and collision avoidance. These integrated systems, combining computational feedforward models with impedance-responsive sensors for real-time feedback, are essential for overcoming flow irregularities and structural stability issues, thereby pushing hydrogel-based AM closer to clinically relevant outcomes.
How this paper relates to my chapter
This review paper connects directly to the ideas in our chapter by showing how the very things that make hydrogels great for medical scaffolds, like being soft, wet, and flexible, also make them challenging 3D print into complex shapes. It explores advanced printing methods and smart computer planning that work with a hydrogel’s natural behavior. The paper provides a real-world example of how understanding the core properties of the hydrogel polymers is the key to building functional, life-like structures for biomedical applications.

