32 Hydrophilicity and Hydrophobicity in Advanced Material Applications
xgong8

1. Introduction to hydrophilicity and hydrophobicity of polymers
Polymeric materials have become an indispensable part of modern industry, medicine and daily life due to their structural diversity and tunable properties. Among all the factors affecting the properties of polymers, hydrophilicity and hydrophobicity are two crucial properties that not only determine the surface behaviour of polymers but also profoundly influence their application potential in various environments. Understanding and regulating the balance between hydrophilicity and hydrophobicity is an important entry point for designing functional polymer materials.
Molecular basis of hydrophilicity and hydrophobicity
Hydrophilicity and hydrophobicity are central properties of polymer surface and bulk phase properties. Hydrophilicity allows the material to interact closely with water molecules and is often used to promote wetting, water absorption or water phase solubility. Hydrophobicity, on the other hand, emphasizes the ability to repel water and is commonly used in water-repellent, water-insulating and oil-repellent coatings. Hydrophilicity and hydrophobicity arise from the difference in polarity of the chemical groups in a polymer molecule. Hydrophilicity is usually caused by polar or charged groups (such as hydroxyl -OH, carboxyl -COOH, and amine -NH2), which strongly interact with water molecules through hydrogen bonding or electrostatic interactions, thus enhancing the polymer’s affinity for water. In contrast, hydrophobicity is determined by nonpolar groups (e.g., alkyl groups -CH3, aryl rings, fluorine-containing groups -CF3), which interact weakly with water molecules, resulting in polymers tending to repel water.
Regulation of hydrophilicity and hydrophobicity
In polymer science, hydrophilicity and hydrophobicity can not only be directly regulated by the design of the chemical structure but can also be optimized by surface treatment, blending or compounding. For example, the addition of hydrophilic functional groups can improve the wettability and biocompatibility of polymers, while the introduction of hydrophobic groups can improve the water resistance and chemical stability of materials. In addition, polymers with both hydrophilic and hydrophobic regions can be prepared through the design of block copolymers, and such amphiphilic structures are widely used in emulsifiers and drug-delivery vehicles.
Hydrophilic and Hydrophobic Applications
The difference between hydrophilicity and hydrophobicity allows polymers to exhibit unique functionality in a variety of applications. Hydrophilic polymers, such as polyethylene glycol PEG, are widely used in medicine for anti-protein adsorption coatings and drug delivery systems, while hydrophobic polymers, such as polypropylene PP, excel in the waterproofing and packaging industries. In addition, by modulating the balance of hydrophilicity and hydrophobicity, it is possible to develop smart-responsive materials such as temperature-sensitive polymers (e.g., PNIPAM) that exhibit reversible hydrophilic and hydrophobic transitions at specific temperatures.
This chapter will systematically introduce the role of polymer hydrophilicity and hydrophobicity in the structure-property relationship from theoretical foundations to application examples, providing readers with a clear framework for understanding and guidance for practical applications.
2. Basic concepts of hydrophilicity and hydrophobicity[1]
2.1 Mechanisms at the molecular level
2.1.1 Chemical groups and molecular polarity
Hydrophilicity arises from strong interactions between the surface of a material and water molecules, usually involving hydrogen bonding, dipole-dipole interactions, or electrostatic attraction. Typical hydrophilic groups include:
Hydroxyl (-OH): binds to water through hydrogen bonding. For example, the high hydrophilicity of polyvinyl alcohol (PVA) is due to the large number of hydroxyl groups in the molecular chain.
Carboxylic group (-COOH): dissociates to form a negatively charged carboxylic acid radical that strongly interacts with water molecules. Polyacrylic acid (PAA) is an excellent water-absorbent resin material due to the large number of carboxyl groups.
Sulfonic acid group (-SO3H): Commonly used to improve the hydrophilicity of conductive materials, such as sulfonated polyether ether ether ketone (SPEEK) in proton exchange membranes.
2.1.2 Hydrophobicity
Hydrophobicity is usually dominated by van der Waals forces of non-polar groups on the material’s surface. These groups repel polar water molecules and form low-wettability surfaces. Common hydrophobic groups include:
Alkyl chains (-CnH2n+1): polyethylene (PE) and polypropylene (PP), for example, exhibit excellent hydrophobicity due to the lack of polar groups in the molecule.
Aromatic rings (e.g. benzene ring): have a stabilized π-electron system that further reduces the adsorption of water molecules. Polystyrene (PS) is a classic case.

2.2 Structure and arrangement of molecular chains
2.2.1 Linear and branched chains
- Linear Chain: Polar groups are evenly distributed, increasing the hydrophilicity of the material. For example, polyethylene glycol (PEG) exhibits extreme water solubility.
- Branched Chains: Hydrophobic groups are clustered within the molecule, decreasing the surface polarity. For example, polystyrene (PS) has branched chains that make it hydrophobic to water.
2.2.2 Crosslinked Networks
- Highly Crosslinked Polymers: A high degree of crosslinking significantly reduces hydrophilicity. For example, the crosslinked network in silicone rubber hinders the penetration of water molecules.
- Low-Crosslinked Hydrogels: Hydrogels with low crosslinking degrees, such as sodium polyacrylate, can absorb large amounts of water, exhibiting remarkable hydrophilicity.
2.3 Surface morphology and roughness
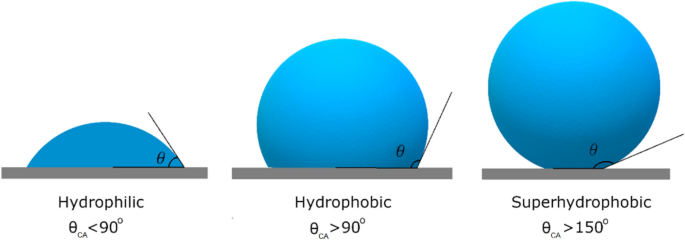
Surface morphology further amplifies hydrophilicity or hydrophobicity by changing the contact angle. The contact angle is a central indicator for assessing hydrophilicity and hydrophobicity. The “contact angle” is the angle formed when a water droplet comes into contact with a solid surface, essentially measuring the degree to which water “wets” the surface; a small contact angle indicates that the surface is hydrophilic (water-loving) and that the water spreads easily; a large contact angle suggests that the surface is hydrophobic (water-repellent) and that the water tends to condense and form beads[3]
Low contact angles (<90°) indicate strong hydrophilicity, such as droplet expansion behaviour on glass surfaces.
A high contact angle (>90°) indicates strong hydrophobicity, e.g. self-cleaning behaviour on the surface of a lotus leaf.
Superhydrophobic surfaces with contact angles exceeding 150° are often realized by building micro- and nanocomposite structures (e.g., micropillar arrays) and low surface energy coatings.
Surface morphology further amplifies hydrophilicity or hydrophobicity by changing the contact angle.
Smooth surfaces: surface groups directly determine hydrophilic/hydrophobic properties.
Rough surfaces: Combining the Wenzel model or the Cassie-Baxter model further enhances the material’s wetting or water-repelling properties through micro- and nanocomposite structures.
Example:
Superhydrophobicity on the surface of a lotus leaf combines micron bumps with nanostructures to achieve contact angles greater than 150°.
3. Regulation of hydrophobicity and hydrophilicity
3.1 Chemical Modification
The most direct method of regulating the surface chemical properties of a material is through chemical modification, which can either increase or decrease its hydrophilicity. This is achieved by introducing specific functional groups or by modulating the interaction between the material and water molecules through surface treatment technology.
Methods and Principles
1. Introduction of polar groups
Polar groups (e.g., hydroxyl -OH, carboxyl -COOH, sulfonyl -SO3H, etc.) can significantly enhance the hydrophilicity of the material.
The hydroxyl (-OH) group can be introduced by forming a hydrogen bond with water molecules, significantly enhancing the material’s hydrophilic properties.
The carboxyl (-COOH) group can be dissociated into carboxylate anions (COO⁻), which further strengthens the interaction between the material and water through electrostatic forces. The difficulty of wetting with water is indicated by the strong hydrophobic or even superhydrophobic nature of the material.
2. Fluorination
The introduction of low surface energy groups (e.g. perfluoroalkyl chains -CF3 or -CF2-) on the surface of a material can significantly improve hydrophobicity. The extremely low surface energy of fluorination makes it difficult for water molecules to wet the surface, resulting in strong hydrophobic or even superhydrophobic properties[5].
Examples of Applications
- Polypropylene (PP), which is a hydrophobic material itself, is converted into hydrophilic film by introducing hydroxyl or carboxyl groups on the surface through plasma surface treatment, which is used for water filtration membranes.
- Polyvinyl alcohol (PVA) is used to prepare highly absorbent gels by grafting carboxyl groups on the surface[6].
- The fluorination of silicone surfaces introduces -CF3 groups, rendering them superhydrophobic coatings suitable for waterproofing electronic devices.
- Fluorinated polymers, such as PTFE and Teflon, are commonly used in non-stick coating and waterproof clothing[7].
3.2 Surface Engineering
Surface engineering involves modifying the surface roughness and nanostructure of materials to significantly enhance their inherent hydrophilic or hydrophobic properties. This method The combination of physical and chemical techniques allows the enhancement of material performance through the construction of surface topography.
Methodology and Principles
1. Etching Technology
Surfaces with micron- or nanometer-scale roughness are constructed by laser, ion beam, or chemical etching. These rough structures amplify the inherent hydrophilicity or hydrophobicity of the material according to the Wenzel and Cassie-Baxter models.
- Wenzel model: Liquid completely infiltrates the rough surface and the roughness increases the hydrophilic or hydrophobic effect.
- Cassie-Baxter model: rough surface forms air pockets, water droplets have reduced contact area with the solid and exhibit superhydrophobicity.
2. Self-assembled monolayer (SAM)
Self-assembled monolayers refer to the chemical adsorption of specific molecules on a surface so that their molecular chains form a highly ordered monolayer structure on the surface. For example, long-chain alkyl mercaptans form SAMs on gold surfaces, which are used to modulate surface energy and polarity.
Applications
- Hydrophilic oxide layers are generated by plasma treatment on glass or silicon surfaces and are used in microfluidic devices to ensure uniform liquid flow.
- Laser etching of aluminum alloy surfaces combined with low surface energy coatings to prepare superhydrophobic metal surfaces for anti-icing and anti-corrosion coatings.
- Self-assembly of silica nanoparticles is utilized to form micro-nano structures for waterproof self-cleaning surfaces that mimic lotus leaves.
3.3 Blending and copolymerization[8]
Amphiphilic materials formed by combining hydrophilic and hydrophobic polymers are capable of combining both properties. Blending and copolymerization methods are the two main ways to realize the design of such materials.
Methods and Principles
1. Polymer Blending
Blending is the physical mixing of two or more polymers to optimize the ratio of hydrophilic and hydrophobic components to achieve the target properties. This method is a simple process but may lead to phase separation phenomena when intermolecular compatibility is poor.
2. Block copolymer[9]
Block Copolymer consists of polymer chain segments with different hydrophilic and hydrophobic properties, such as alternating hydrophilic segments (e.g., polyethylene glycol PEG) and hydrophobic segments (e.g., polylactic acid PLA). Block copolymers not only improve molecular compatibility, but also form specialized nanostructures through self-assembly.
Applications
Drug delivery[10]:
PEG-PLGA block copolymers form aqueous gel-like structures. The hydrophilic PEG forms an outer shell that is water-soluble, while the hydrophobic PLGA forms an inner core. These materials are used to encapsulate hydrophobic drugs. The advantages of this method are that the release of the drug can be controlled and that the material is biocompatible.
Emulsifying and Stabilizing Agents:
Two-component block copolymers (such as PEO-PPO-PEO) are used as emulsifying agents in the cosmetics and food industries. The advantage of this method is that it forms stable emulsions at oil/water interfaces.
4. Literature Review of Hydrophilic Polymers: Synthesis, Self-Assembly, and Applications[11]
Hydrophilic polymers are an important type of polymer. They are used in many areas, from superabsorbent materials to drug delivery. This review looks at recent progress in this field. It focuses on common types of hydrophilic polymers, how they self-assemble, and how they are used to make particles.
Hydrophilic Homopolymers
Hydrophilic polymers are grouped based on the composition of their backbone. Some of their main applications include:
- Wastewater treatment: Polymers like polyacrylic acid (PAA) or cationic polymers like PDADMAC are used to help remove impurities by flocculation.
- Biomedical applications: Glycopolymers are often used because they mimic natural sugars. This helps in biological processes like cell adhesion and fighting infections.
Recent studies have focused on these hydrophilic polymers:
- Poly(carboxybetaine methacrylamide) (PCBMAA): Made using RAFT polymerization to achieve high molecular weights.
- Poly([2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide) (PSBMA): Researchers used NMR to study how it interacts with water.
- Polyethylene glycol (PEG): PEG is widely used but has issues with anti-PEG antibodies. New options like polyglycerols and other alternatives are being tested.
- Poly(sarcosine): This polymer is easy to make and is being studied for its behavior in solution.
Grafting Hydrophilic Polymers
Attaching hydrophilic polymers to surfaces can add useful properties:
- Anti-fogging: Coatings with PCBMAA can prevent fogging.
- Lubrication: Polymers like DMAEMA, MPC, TAC, and SPMK are being tested for their slipperiness when applied to silicon wafers.
- Anti-biofouling: Polymers like PSBMA and PCBMAA are being studied for their ability to resist biological buildup, like on medical devices.
- Antimicrobial effects: Polymers with lysine or similar structures are being explored to kill harmful microbes.
Aqueous Two-Phase Systems (ATPS) and Water-in-Water (w/w) Emulsions
- ATPS and w/w emulsions are made from polymers like PEG and dextran. They create oil-free and biocompatible environments.
- Researchers are studying how these systems form and what affects their behavior, such as molecular weight and polymer concentration.
Specific applications include:
- Using PEG and dextran systems to separate molecules into different phases. For example, fibrous assemblies move to the dextran-rich phase.
- Making ATPS at lower concentrations using very high molecular weight polyacrylamides.
- Improving biosensing by concentrating DNA or other molecules in one phase makes detecting them easier.
- Creating stable w/w emulsions for delivering substances or forming compartmentalized hydrogels.
Self-assembly of Hydrophilic Polymers
- Hydrophilic block copolymers can self-assemble in water to form structures like micelles, capsules, or particles.
- Scientists are studying how to control this process by changing the ratio of blocks, concentration, or external conditions.
Examples include:
- Pullulan and poly(acrylamide) block copolymers were used to create vesicles. These combine synthetic and natural polymer properties.
- Studying how certain copolymers form larger, separated phases in concentrated solutions.
- Stabilizing self-assembled structures with hydrogen bonding using molecules like tannic acid.
Stimuli-Responsive Polymers
Some hydrophilic polymers respond to triggers like temperature, pH, or light. This lets them change structure or release cargo when needed, making them useful for drug delivery.
Examples include:
- Temperature-sensitive copolymers: These form different structures depending on temperature.
- pH-sensitive copolymers: For example, PMPC-b-PDIAEMA forms vesicles in acidic conditions and micelles at higher pH.
- Redox-sensitive copolymers: These can break down under specific conditions to release their cargo.
Hydrophilic Polymer Particles
Hydrophilic polymers can form particles like hydrogels or coacervates. Their properties depend on how they are made, such as through crosslinking or precipitation.
Applications include:
- Stable particles: These resist dilution and are used for enzyme storage and controlled release.
- Coacervates: Made from oppositely charged polymers, they are used in bioseparation and drug delivery.
- Single-chain nanoparticles: These imitate how proteins fold and have potential for protein-like functions.
Future Directions
The review points out areas for improvement in hydrophilic polymer research:
- Discovering new polymers with unique properties.
- Developing greener and more efficient ways to make them.
- Expanding their use in drug delivery and other biomedical fields.
- Understanding how to better control self-assembly.
- Finding ways to degrade these polymers in an environmentally friendly way.
Conclusion
Hydrophilic polymers are versatile and continuously evolving. Research is focused on making them better and finding new uses, especially in medicine, cosmetics, and food. Future work will aim to create biodegradable and biocompatible materials and solve current challenges.
- Bayliss, N., & Schmidt, B. V. K. J. (2023). Hydrophilic polymers: Current trends and visions for the future. Progress in Polymer Science, 147, 101753. https://doi.org/10.1016/j.progpolymsci.2023.101753 ↵
- Guo, X., Wang, L., Wei, X., & Zhou, S. (2016). Polymer-based drug delivery systems for cancer treatment. Journal of Polymer Science Part A: Polymer Chemistry, 54. https://doi.org/10.1002/pola.28252 ↵
- Jhaveri, J. H., & Murthy, Z. V. P. (2016). A comprehensive review on anti-fouling nanocomposite membranes for pressure-driven membrane separation processes. Desalination, 379, 137–154. https://doi.org/10.1016/j.desal.2015.11.009 ↵
- https://link.springer.com/article/10.1007/s10999-022-09593-x ↵
- Alves, A. V., Tsianou, M., & Alexandridis, P. (2020). Fluorinated Surfactant Adsorption on Mineral Surfaces: Implications for PFAS Fate and Transport in the Environment. Surfaces, 3(4), 516-566. https://doi.org/10.3390/surfaces3040037 ↵
- Raza, Z.A., Khatoon, R., Banat, I.M. (2021). Altering the Hydrophobic/Hydrophilic Nature of Bioplastic Surfaces for Biomedical Applications. In: Kuddus, M., Roohi (eds) Bioplastics for Sustainable Development. Springer, Singapore. https://doi.org/10.1007/978-981-16-1823-9_17 ↵
- Brunn, H., Arnold, G., Körner, W., et al. (2023). PFAS: Forever chemicals—persistent, bioaccumulative and mobile. Reviewing the status and the need for their phase-out and remediation of contaminated sites. Environmental Sciences Europe, 35(20). https://doi.org/10.1186/s12302-023-00721-8 ↵
- Khan, I., Mansha, M., Jafar Mazumder, M.A. (2019). Polymer Blends. In: Jafar Mazumder, M., Sheardown, H., Al-Ahmed, A. (eds) Functional Polymers. Polymers and Polymeric Composites: A Reference Series. Springer, Cham. https://doi.org/10.1007/978-3-319-95987-0_16 ↵
- (2007). Polymer Blends and Block Copolymers. In: The Physics of Polymers. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-68411-4_4 ↵
- Guo, X., Wang, L., Wei, X., & Zhou, S. (2016). Polymer-based drug delivery systems for cancer treatment. Journal of Polymer Science Part A: Polymer Chemistry, 54. https://doi.org/10.1002/pola.28252 ↵
- Bayliss, N., & Schmidt, B. V. K. J. (2023). Hydrophilic polymers: Current trends and visions for the future. Progress in Polymer Science, 147, 101753. https://doi.org/10.1016/j.progpolymsci.2023.101753 ↵