27 Plasticizers and their Effects
Reid Barnett
Learning Objectives
Upon completion of this chapter you will be able to:
- Explain the impacts of plasticizers on polymer properties and structure.
- Describe the mechanism of leaching and how it applies to plasticizers.
- Explain unintended impacts of plasticizers and how to mitigate them.
- Analyze various plasticizers to determine best fit for a use case.
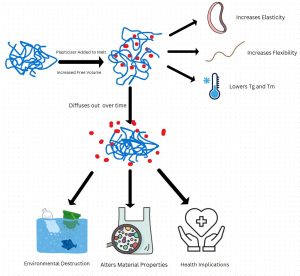
1.0 Introduction to Plasticizers
When polymers are used in real world applications, they are rarely used in their pure form. Polymer properties can be augmented by the inclusion of additives in the polymer melt or solution. There are many different classifications of additives, differentiated based on which properties they are designed to affect. Plasticizers are an extremely common example in the polymer industry, and are used to affect the mechanical properties of a polymer [1]. Oftentimes, polymers need to be softer, more pliable, or tougher than they are usually capable of to fulfill the technical or design specifications for a particular product. This change in properties is related to how the plasticizers interact with the amorphous regions of the polymer, and causes a massive change in the overall mechanical properties of a polymer.
1.1 Microstructure
Plasticizers affect the microstructure of amorphous and semicrystalline polymers to achieve the desired properties. All polymer materials have amorphous regions that are disorganized and inefficiently packed, allowing some degree of flexibility. These amorphous regions have free space for polymer chains and polymer chain segments to move around and slide past one another. Plasticizers, and other additives, inhabit the amorphous regions of the polymeric material and increase the available free space by repelling the polymer chains and giving them something easier to slide across [1]. The addition of plasticizers to the polymer melt increases the size of the amorphous regions and decreases their density, but the plasticizers do not work directly on the crystal structure of the polymer. The crystal regions of the polymer are packed too tightly for the plasticizer to penetrate, but the addition of plasticizers to the melt impedes the ability for crystals to form and so crystallinity decreases as plasticizer content increases [2]. This leads to many alterations to the typical properties of a polymeric material.
1.2 Properties
The changes in polymer microstructure from the inclusion of plasticizers are the driving force behind the changes in the bulk mechanical properties exhibited by a polymer. As the plasticizer concentration increases, the free volume of the amorphous region increases; there is a decrease in stress at break and surface hardness and an increase in the strain at break [3]. These property changes are highly desirable for certain applications and increase the overall usability of polymers for a wide range of uses. PVC is an excellent example of the power of plasticizers to change how a material behaves. Pure PVC is usually hard, brittle, and inflexible, used for pipes to transport water or wastes, but when it has been processed with plasticizers it can become flexible enough to make shower curtains and flexible tubing [4]. Without plasticizers, many of the applications that we associate with polymers would be either impossible or far more difficult to achieve.

2.0 Basic Concepts of Plasticizers
Plasticizers are a broad classification of substances with varied chemical makeup, but they all share one important trait, they are all designed to increase the flexibility of polymeric materials. They accomplish this through a similar set of mechanisms that affect the microstructure and intermolecular interactions between polymer chains. These changes affect the polymer on a multitude of levels, from the mechanical and thermal properties of the final product to the rheological properties of the melt during processing [1]. Plasticizers are invaluable tools for engineers and scientists to manipulate the properties of polymeric systems for both the desired end products and the ease of processing those goods. These plasticizers must also be compatible with the polymers they will be used with, with polar plasticizers weakening the intermolecular forces of polar polymers very well and nonpolar plasticizers increasing the free volume in a nonpolar polymer very well [1]. With all of these ideas in mind plasticizers are an invaluable addition to the polymer industry that needs to be well understood to fully utilize their capabilities.
2.1 Mechanical effects on polymers
Plasticizers are designed specifically to alter the mechanical properties of polymeric materials, usually specific polymer types such as PVC, PLA, or Polyester. The inclusion of plasticizers into the polymer melt affects many of the properties of the polymer in both melt and solid phase, and in both the crystalline and amorphous regions of the polymer. The addition of plasticizers can affect the properties of polymers in a myriad of ways from reducing the Young’s modulus and increasing tensile strength [5], to altering the crystallization state of the polymer by altering the free energy of the system to either increase or decrease crystallinity depending on type of polymer and plasticizer and concentration [2]. These varied changes of properties are due to underlying alterations in the polymer microstructure that affect all parts of the polymer and result in a material or product that does not even closely resemble the pure polymer structure in some cases.
2.1.1 Amorphous Domain
The amorphous region of the polymer is where plasticizers have the greatest impact on the overall structure and properties of a polymer. The plasticizers, while typically smaller molecules, are too large to fit between the polymer chains in the crystal region, but can easily inhabit the chaotic and more open space within the amorphous domain, even going so far as to be concentrated in the amorphous region as the crystals grow and expel the plasticizers from those areas [2]. Plasticizers act in the amorphous region by increasing chain movement and reducing the intermolecular forces that act between polymers in the amorphous regions. This reduced attraction between chain segments allows them to move past each other with far more ease, which makes the polymer more flexible and ductile. The freer chain segments in the amorphous region also make it harder for crystals to grow and so increase the amount of the polymer chain in the amorphous region [2]. The plasticizers increase the free volume and free energy of the polymeric material in the amorphous region which is the underlying driving force of most of the impacts that they cause in polymeric materials.
2.1.2 Crystalline Domain
Plasticizers may not directly affect the structure of polymer crystals in most cases, they are certainly responsible for many indirect effects on their size, quantity, and quality. Polymer crystals are formed from the amorphous polymer being packed more neatly and orderly, but only in the circumstances where the transition from disordered amorphous polymer to orderly polymer crystal is energetically and kinetically favorable to do so. This process is disrupted by the addition of plasticizers which increase the freedom of motion that polymer chain segments have, thus making it more difficult for them to be confined into an orderly and compact crystal structure [2]. This can reduce the crystallinity of the polymer and be a restraint on higher percentage crystallinity. As the crystallinity of the polymer increases the plasticizer is concentrated further in the amorphous regions surrounding the crystal and makes any further growth of the crystal harder, increasing the plasticizer’s effects on the amorphous region proportionally to its concentration [2]. This dynamic interplay between the crystallinity of a polymer, the local concentration of the plasticizer, and the impacts of the plasticizer on the amorphous regions surrounding the crystalline regions makes for very interesting relationships that changes the balance from where it would be for a pure polymer and into new territory.
2.1.3 Bulk Properties
The bulk mechanical properties of polymers is usually the main reason for the use of plasticizers. The aforementioned impacts on both the crystalline and amorphous domains of the polymer by plasticizers leads to a few changes in the bulk behaviors of the resulting materials. The most obvious of these changes is that the polymer becomes more “plastic”, meaning that it has become more flexible, ductile, and deformable [6]. These characteristics are often very desirable and arise due to both the higher mobility of the polymer chains in the amorphous regions and the lower percent crystallinity in conjunction with one another. It is the microstructural changes made by the plasticizers onto the polymer that directly results in these alterations in the bulk mechanical properties of the polymer.
2.2 Thermal Effects of Plasticizers
The physical and microstructural alterations of the polymer from the inclusion of plasticizers also greatly impacts its thermal phase transitions. As a useful rule of thumb, as the concentration of plasticizer increases the thermal transition temperatures decrease. This is due to the higher chain mobility associated with higher concentrations of plasticizer, giving them more degrees of freedom [3]. The thermal effects of plasticizers are also typically desirable to increase the ease of processing and to reduce energy expenditure when processing the polymer.
2.2.1 Glass Transition Temperature, Tg
The glass transition temperature is the most important when discussing the use and value of plasticizers. Plasticizers can reduce the Tg below certain temperatures, which can allow for polymers to expand their working temperature range, or at least shift it towards a more desirable range for specific applications. Many polymers, like PVC naturally have a Tg higher than room temperature and so can be brittle or glassy in many environments when in their pure form, but the inclusion of plasticizers allows the Tg to be pushed below standard operating temperatures to maintain a rubbery behavior in those environments [7]. This rubbery phase of semicrystalline polymers is often the most desirable, where the material has high strength and toughness without compromising on flexibility, so the ability to expand that rubbery temperature range opens many opportunities for polymers.
2.2.2 Melt Temperature, Tm
Lowering the melt temperature is often an impact of higher concentrations of plasticizers. When polymers are being processed they must be above their melt temperature so that there are no crystals and the polymer can flow like a fluid, which is a significant energy expenditure in the processing of polymers [2]. The plasticizer concentration makes it easier for crystals to melt by providing more degrees of freedom, which reduces these energy needs during processing and thus increasing overall efficiency.
3.0 Secondary Impacts of Plasticizers
Beyond the intended uses of plasticizers for the manipulation of physical and thermal properties of polymeric materials, plasticizers have many unintended outcomes from changes in performance over time to adverse health and environmental effects from both plasticizers and their metabolites [8]. These impacts will be explored both through their physical and chemical mechanisms of dispersion and the biological impacts after the fact. Plasticizers have become almost ubiquitous in the environment, not in small part to the tens of millions of pounds used every year for PVC alone [9]. This large volume and long lifespan in the environment makes the understanding of plasticizer impacts very important.
3.1 Migration
Migration is the physical process by which the plasticizer moves from the desired polymer into another solid material it is in contact with. Plasticizers are not chemically bonded into the structure, and so they have the freedom to move around within the material and into other nearby materials [7]. This is determined by a few factors such as the compatibility of the plasticizer to the other polymer, plasticizer concentration, time, and molecular weight of the polymer [7]. This is often a negative outcome of plasticizers and can contaminate foods and alter nearby materials in undesirable ways which could lead to failure.
3.2 Leaching
Leaching is the process by which plasticizers are released by a material into a liquid, typically water, which is the most concerning for both health and environmental reasons. The environmental impacts of leaching are seen most acutely from microplastic pollution in the ocean and other surface waters, specifically looking at phthalate and phenol plasticizers which are incredibly common and have been known to be toxic [6]. Leaching is very strongly influenced by the physical environment that the material is located in, including the motion of particles in waves and moving water, the particle or part size, and the temperature [6]. There are chemical structures that plasticizers can be given to reduce their leaching, but it is not completely stopping the problem. By incorporating benzene rings, branched oligomers, or longer chains in the plasticizers the issue of leaching can be reduced, but it will still be necessary to replace harmful plasticizers with more environmentally friendly options [4]. The industry is slow to change because of the lack of options which can achieve the same functionality as toxic leaching options at a similar price point.
3.3 Biological Interactions
While plasticizers are incredibly valuable tools for engineering polymer systems and tuning their properties, they are also known to cause massive environmental damage as well as lead to undesirable health outcomes. When plasticizers enter the body or the environment, it is not just the initial chemical that needs to be considered, but also any metabolites or byproducts that are created as it degrades. Phthalates are one of the main culprits when it comes to environmental degradation for plasticizers, leading to disease and death for aquatic plants and animals when its concentration exceeds normal levels, and is also a known carcinogen and endocrine disruptor [8]. It is of paramount importance to recognize that these plasticizers are negatively impacting not just the environment but also human health in a real and tangible way, and should be excluded as much as possible. The engineering and scientific design process does not end at the final product, but rather at the point at the end of that product’s life cycle and should include the full scope and breadth of the impacts that it will have on the wider world at large.
4.0 Current Research
https://doi.org/10.1016/j.scitotenv.2022.159099
This paper shows where a good portion of the research in the field of plasticizers is looking, the environmental impacts and driving forces. It is important to understand the mechanisms by which plasticizers enter the environment in order to understand how they may be influencing the environment and how they may be removed. This paper explores the factors that impact leaching rates of plasticizers from polystyrene microplastics in the environment, specifically the most common and toxic varieties, diphenols and phthalates. These different plasticizers, diphenol and phthalates, behaved radically different in their leaching dynamics and were affected differently by variables. The experiments showed that diphenols leaching dynamics were very dependent on time and temperature, exhibiting behaviors of a free volume core, which limits the rates of diffusion of the plasticizers because they are more evenly distributed through the bulk of the polymer. The phthalates, on the other hand, showed almost all of their leaching occurred during the initial rinse due to them being mostly concentrated on the surface and their immiscibility in water. Phthalate plasticizers also did not show much dependence on temperature. However, despite the differences in the impact of other variables, both classes of plasticizers exhibited higher leaching rates with increased wave energy in the system.
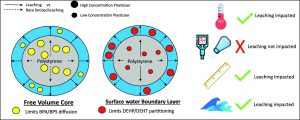
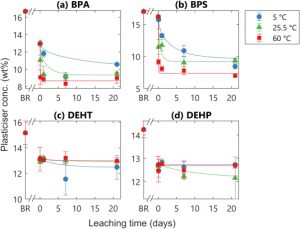
This paper shows the importance of designing plasticizers with an eye towards their full life cycle. Environmental degradation is an issue that everyone faces, but without proper understanding of the background causes it can be difficult to stop or fix. By understanding how plasticizers behave in aquatic environments it is possible to design the next generation of materials and plasticizers that rectify the issues and the systems that prevent this pollution from entering the environment in the first place. It is necessary to plasticize the PS for the desired properties and applications, but now is time to explore new materials and processes that may be able to achieve similar results without the attached environmental and biological devastation that many of the more traditional plasticizers are known for.
