10 Solubility parameters of polymers
sali8
Learning Objectives
By reading this chapter, the reader will be able to:
To learn the basic concepts/theories for polymer solubility in the solvents
To introduce some mathematical calculation for quantifying the polymer solubility
To get an overview of a polymer (i.e., PEG) solubility in water, DMSO, chloroform, THF, and methanol
Graphical Abstract
Introduction:
Solubility of a substance means complete or partial dissolving of that substance in a suitable solvent and the solution looks complete or partial miscibility. In other words, further chemical separation process would be required for separating the substance from the solvent. To execute the chemical reaction or chemical synthesis, solubility is one of the key properties, as this solubility would promote more feasible chemical reaction, including energy transformation smoothly for proper chemical substance synthesis. The solubility of a substance is dependent on several factors: thermodynamics of the solution, intermolecular forces, hydrogen bonding, heat of mixing, cohesive energy, polymer crystallinity and amorphousness (1).
This solubility is taken consideration for manufacturing different products, like paintings, spinning fibers where different types of monomers are dissolved into suitable solvents for proper mixture and enhancing the fiber spinnability, plasticization of polymers, textile dye synthesis, and casting films. In terms of polymers, this parameter holds significance as there are several types of polymerization process where each monomer needs to be put into the suitable solvents and then polymerization reaction is done. Apart from that, this solubility largely dependent on the monomer’s chemical structure, incorporating catalyst, and solvents chemical construction (1). Adding to that, this solubility might differ when a mixture of solvent is used rather than single solvent for that certain monomer (2).
Among several factors considering the solubility, cohesive properties are directly related to polymer solubility in organic solvents. Quantification of this cohesive property is known as cohesive energy. This quantity is closely related to the internal pressure of the solvent, a parameter appearing in the equation of state of the substance. In general, the solubility of each polymer in a solvent is solely dependent on its chemical structure as well as the chemistry of the solvent. The solubility parameter of the polymer is always defined as the square root of the cohesive energy density in the amorphous state at room temperature. Besides the chemical structure, also the physical state of a polymer is important for its solubility properties. Crystalline polymers are relatively insoluble and often dissolve only at temperatures slightly below their crystalline melting points, and this is due to their lack of excess free energy in the crystalline region (2).
Theories for solubility:
Cohesive energy:
In general, cohesive energy is considered the minimum required energy involves that can separate individual atom of a substance from its surrounding atoms in a particular medium. The cohesive energy Ecoh of a substance in a condensed state is defined as the increase in internal energy U per mole of substance if all the intermolecular forces are eliminated:
The cohesive energy Ecoh= ∆U (dimension: J/mol)…………………………………………………………. (2)
This understanding of cohesive energy can lead to evaluating the solubility parameter as well as cohesive energy density which can be useful for understanding the solubility of a polymer in a suitable solvent.
The cohesive energy ҽcoh= Ecoh/V (dimension: J/mol). (at 298 K) in: J/cm3 or MJ/m3 or Mpa
Solubility parameter δ= (Ecoh/V)1/2 = (ҽcoh)1/2 (at 298 K) in (J/cm3)1/2 or (MJ/m3)1/2 …(2)
Impact of chemical composition group on cohesive energy:
As for low molecular weight substances, Ecoh was considered as an additive property, and it was derived group contributions for the cohesive energy of liquids at room temperature. The values given by the different authors show a rough correspondence. Since the cohesive energy is reciprocal proportional to increase temperature, below condition is satisfied in most of the cases:
H00 > Ecoh (298) >Ecoh(Tb) ………………………………………………………………..(2)
Where, Ecoh(Tb) is the cohesive energy at boiling temperature,
Ecoh(298) is the cohesive energy at 298K, and
Ho0 is the cohesive energy at 0K
For example, if there is a -CH3 group in the low molecular weight of a polymer, then its cohesive energy would be 10560 J/mol, 4150 J/mol, 7120 J/mol at 0K, 298K, and boiling temperature K. Here, we can understand that the previously mentioned relationship is broken. However, this cohesive energy can be used for quantifying the solubility of a polymer in a solvent by considering the presence of functional group (2).
The determination of Ecoh:
For liquids of low molar weight, the cohesive energy is closely related to the molar heat of evaporation
Ecoh= ∆Uvap= ∆Hvap – p∆V
From that aspect, it can be said that by calculating the required amount of heat energy for evaporation, the cohesive energy can be calculated for low molar weight chemicals. On the other hand, as it would be difficult to evaporate polymer, in that case indirect methods must be used for the determination of their cohesive energy, e.g. comparative swelling or dissolving experiments in liquids of known cohesive energy density (2).
Thermodynamic of solution:
In terms of mixing two components, it would be more practical to dissolve the mixture by considering the free mixing energy is in negative value. It can be measured from the partition function of the mixture. Huggins had given an equation for measuring the energy required for mixing components, and it is shown below:
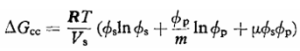 …………………………………………………………….(1)
…………………………………………………………….(1)
Where, ∆Gcc is the free energy/c.c. of mixture, ɸ is the volume fraction (subscripts s and p refer to solvent and polymer respectively) and Vs is the molar volume of solvent; m is the ratio of molar volumes of polymer and solvent, so that mVs is the molar volume of the polymer. The constant p is the sum of two parts,
![]() ………………………………………………………………………………(1)
………………………………………………………………………………(1)
µz is a small constant (empirically 0.2-0.3) depending upon the co-ordination number of the quasi-lattice assumed in the entropy calculation, and K is a heat of mixing constant for the system concerned by
![]() …………………………………………………………………………………….(1)
…………………………………………………………………………………….(1)
Where, ∆Hcc is the heat of mixing/c.c. of mixture.
Intermolecular forces:
The electrostatic force arising between two molecules of a compound that attracts or repulses the two molecules – known as intermolecular forces. In case of attracting, two molecules come together and convert the compound stable, whereas in case of repulsing, the opposite phenomenon can happen. These intermolecular forces are important for chemical substances, as these forces act as a driving force for solubility in the suitable solvent, viscosity and enthalpy.
Among different intermolecular forces, dispersion force is one of the significant forces that allow the molecules to be attached in a suitable solvent. In general, this force is also known as van-der-walls force and due to its weak force, this force is considered as a temporary force between the two molecules. Adding to that, this force is temperature sensitive, and it depends on the dipole nature between the two molecules (1).
Hydrogen bonding:
There is a special type of bond that can be observed when a solute is partially or completely dissolved in the solvent – which is known as hydrogen bond. Typically, this bond can occur between two molecules, when one molecule shows tendency to withdraw electron temporarily from the neighborhood molecules which also showing intention to donate electron. In this way, an inductive effect between the two molecules rose and resulting in positive and negative electrostatic nature between those molecules. Thus, the hydrogen bonding is created due to electrostatic attraction resulting by electron distribution, and this bond allows to stick together two adjacent molecules together and seems like they are soluble with each other mutually (1).
On the other hand, when two adjacent molecules are closer together and their orbitals are saturated by overlapping the electron, then there a slight repulsive force that started to work on due to the saturation of the orbitals. In general, when two chemical components are coming together due to their partially positive and negative sites, allowing the hydrogen bonding – increases the potential energy between those two adjacent chemical components. In the case of the saturation of orbitals by overlapping electrons between the two chemical components, this repulsive force arises but due to their distance decreasing, their potential energy still increases. Besides, this repulsive force and van-der-walls force are always present in between the two chemical components when they are slightly or completely soluble in the solution (3).
Impact of crystallinity:
Previously mentioned all possible forces and factors are relatable to the chemical components, especially polymers that are in liquid state. In terms of solid polymers, there is another theory that needs to be discussed for understanding the solubility in a suitable solvent – polymer’s amorphousness or crystallinity. On order to evaluate the solubility of solid polymer, it is important to know the transition point of that polymer, and under this point polymer is thermodynamically unstable (i.e., glass or supercooled liquid), and when this solid polymer is in contact with a solvent and starts to absorb then this transition point starts to decrease. In this way, more excess energy is started to liberate and promotes to swell the solvent (4).
In the case of amorphous region containing polymer has more excess free energy in its structure due to not packing the polymeric chains tightly and allowing them to dissolve into the suitable solvent. On the other hand, the solid polymer has more crystalline region and due to dense packing polymeric chains in the crystalline region, they do not contain any excess free energy, and that is why supplying more excess energy is required to dissolve the polymer in the suitable solvent. If the polymer is much below its melting point, this free energy will be considerable and the entropy of mixing will not suffice; crystalline polymers are thus, as a rule, appreciably soluble (much below their melting points) only in solvents that have some specific interaction with them, other than dispersion forces, which can only give a positive ∆H of mixing (1).
Discussion of a peer-reviewed journal:
In this chapter, discussed on understanding the solubility of Polyethylene glycol (PEG) by emphasizing on a peer-reviewed journal: “Solubility profiles of poly(ethylene glycol)/solvent systems, I: Qualitative comparison of solubility parameter approaches” which was published in a European Polymer Journal (5).
Overview of the paper:
To understand the solubility of PEG, this paper focused on creating several solubility profiles in different solvents (i.e., water, methanol, chloroform, dimethyl sulfoxide (DMSO) and tetrahydrofuran (THF)). The solubility parameter for PEG in different solvents was evaluated by different algorithmic approaches (i.e., atomic group contribution methods; Small, van Krevelen–Hoftyzer (VKH), Hoy and Breitkreutz methods). Using the solubility parameter theory and equation, 2D and 3D graphs were drawn for understanding the solubility of PEG in different selected solvents (5).
This paper focused on using the solubility parameters by including H-bonding, polar force and van der walls force. Adding to that, PEG polymer’s functional group contribution to cohesive energy density and total solubility parameters by following Small’s approach in the selected solvents were calculated. Finally, the dissolving capability of these solvents has been discussed. In addition, the solubility parameters have been calculated by use of the van der Waals volume in the selected molecule or repeating unit of the polymer instead of the molar volume which is used in atomic group contribution methods. From all the graphs and solubility parameter calculation of PEG in the selected solvents, THF and chloroform solvents would be the best options for solubilizing PEG comparing to methanol, DMSO, and water solvents (5).
Polymer solubility parameter from the peer-reviewed journal connecting to the solubility theory:
The peer-reviewed journal was chosen as this journal used several equations for solubility understanding of a polymer (i.e., PEG) and key concepts and theories for illustrating the solubility of a polymer in a suitable solvent – which were also discussed in this chapter in introduction and theories portions. Below in details the similarities were discussed point-by-point wise:
1.In the theoretical background knowledge, this paper (5) illustrated the solubility parameter by considering the cohesive energy concept and addressed the equation mentioned in the solubility introduction part,
Solubility parameter δ= (Ecoh/V)1/2 = (ҽcoh)1/2 (at 298 K) in (J/cm3)1/2 or (MJ/m3)1/2 ……………..…(2)
2.Adding to that, the peer-reviewed paper (5) included several factors (i.e., hydrogen bonding, polar forces, and van der walls force) for more deep understanding the solubility of PEG in different solvents. The solubility parameter equation extended and formulated by considering these three forces in the paper (5),
Total solubility (δT)2 = (δh)2 + (δd)2 + (δp)2 …………………………………………….(5)
Where, all the solubility parameters were included by considering H bonding (h), dispersion or van der walls force (d), and polar force (p). This chapter also discussed the concept of hydrogen bonding and van der walls force in the introduction part for relating the solubility of polymer in different solvents. Using the solubility parameters for three concepts, total solubility parameter for PEG in all the selected solvents were calculated individually and then 2D and 3D graphs were generated for more visual understanding.
Link of the chosen peer-reviewed journal:
Interactive elements:
In this part of the chapter, several quiz questions were added for self-evaluating the basic concepts of the solubility of a chemical component in a solvent.
YouTube video link explaining the solubility:
Below a link is provided for understanding the basic concepts for solubility of a chemical component in a solvent.
(14) Polymers in Solvents – YouTube
Conclusion
In terms of polymer solubility, there are several aspects that impact on polymer solubility in a solvent. In general, cohesive energy, polymer crystallinity, hydrogen bonding, chemical composition of the polymer and intermolecular forces can vary the polymer solubility. In this chapter, several equations were discussed related to polymer solubility that can measure the solubility parameters of a polymer in a suitable solvent. Adding to that, a peer-review paper discussed on the study of PEG solubility in different solvents and also explained the aspects which were also discussed this chapter. After completion of reading this chapter, a video link and an interactive quiz were also added so that reader can understand the key elements for polymer solubility and can test them about their understanding on polymer solubility.
References:
- PA. Small. Some factors affecting the solubility of polymers. J Appl Chem. 1953;
- Van Krevelen DW, Te Nijenhuis K. Cohesive Properties and Solubility. Vol. i, Properties of Polymers. 2009. 189–227 p.
- Yuan YF, Zhang JM, Zhang BQ, Liu JJ, Zhou Y, Du MX, et al. Polymer solubility in ionic liquids: Dominated by hydrogen bonding. Phys Chem Phys. 2021;23(38):21893–900.
- Moseson DE, Taylor LS. Crystallinity: A Complex Critical Quality Attribute of Amorphous Solid Dispersions. Mol Pharm. 2023;20(10):4802–25.
- Özdemir C, Güner A. Solubility profiles of poly(ethylene glycol)/solvent systems, I: Qualitative comparison of solubility parameter approaches. Eur Polym J. 2007;43(7):3068–93.
