3 Step-Growth and Chain-Growth Polymerization
Isabel Fricke
Introduction
Polymers are essential to life and an integral part of the world around us. Polymers be natural, for example proteins, or synthetic, such as plastics for packaging. Polymers are essential to life and innovation. They are used in many industries, from plastic parts to biomedical devices 1,2. In order to understand these complex molecules, we must first understand how they are synthesized. There are many different ways to synthesize polymers, usually based on what the building blocks are and their characteristics. Polymers are synthesized by smaller molecules that repeat in long chains 1,2. There are two main types that will be discussed in this chapter: chain-growth, or addition, polymerization and step-growth, or condensation, polymerization. These terms are used interchangeably throughout all polymer resources and literature. The key difference is how the monomers come together to form a high molecular weight chain. There are other types of more complex polymerization, but will not be covered in this chapter.
To be considered a polymer, a molecule must be over 10,000 g/mol. It also must be made up of a small repeat unit, originating from a monomer, which combine to form a long, high molecular weight chain 2. Long chains are formed through the formation of stable covalent bonds, resulting in strong and long-lasting materials. Polymers are diverse materials, and can be synthesized in many different ways. They can also be manipulated to change their properties, for a specific purpose. Because polymers can be synthesized through many routes and modified to change properties such as rigidity, flexibility, strength, and thermal stability, understanding how these chains are created is essential for designing materials for specific applications.
Learning Objectives
Upon completion of this chapter, students will be able to:
- Explain the basic mechanisms of chain-growth and step-growth polymerization
- Compare and contrast chain-growth and step-growth polymerization
- Recognize and understand various terms used to describe polymerization
- Appreciate the importance of polymers to everyday life.
Figure 1: Crash Course Chemistry: Polymers
Polymerization
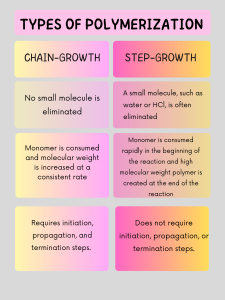
Step-growth Polymerization
Step-growth polymerization is a type of polymerization where a polymer is created, but typically with the loss of a small molecule. This molecule is most commonly water, but can be other small molecules such as HCl or ammonia 2. In this type of polymerization, repeat units containing active sites will bind together to make a dimer (two monomers), trimer (three monomers), or oligomers (many monomers) 1,2. These oligomers then bond to form a long, high molecular weight chain, or polymer. Monomers are consumed early in the reaction, but high molecular weight chains are not formed until the end of the reaction. This reaction occurs without the use of an initiator or a termination step, as the dimers, trimers, or oligomers can react with each other 1,2.
However, a small molecule is not always lost in creation of these polymers. One example is polyurethane, which is a step-growth polymer, but does not lose a small molecule during the reaction, as shown in Figure 2 3. Because of these special cases, the International Union of Pure and Applied Chemistry, or IUPAC, has split step-growth polymerization into two categories: polycondensation and polyaddition. Polycondensation step-growth polymers form and eliminate a small molecule during the reaction process. Polyaddition step-growth polymers do not form or eliminate a small molecule during the reaction process. Figure 2 shows the mechanism of step-growth polymerization 3.
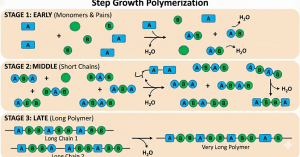
Figure 2: Step-growth polymerization mechanism; created using Nana Banana Pro in Gemini
Chain-growth Polymerization
Chain-growth polymerization a type of polymerization where monomers are added one-by-one to a polymer chain, without the loss of a small molecule. There are three steps to this type of polymerization: initiation, propagation, and termination. Initiation is what makes a reactive group and starts the reaction. This step is essential to the formation of polymer, as without it, monomers would not react with each other 1,2.
Propagation is the step where monomers are added to the reactive end of the growing chain, to create a high molecula weight molecule. This is what allows a chain to grow to a large enough size to be a polymer. Since this is chain-growth polymerization, no small molecule is lost in this process 1,2.
Termination is the final step of chain-growth polymerization. This is the step that makes the chain nonreactive and ends the growth of the polymer. This is the final step, and is vital if you do not want the chain to continue to grow 1,2.
Chain-growth polymerization creates polymers gradually, and the molecular weight will grow continuously throughout the reaction. There are different types of chain-growth polymerization, depending on the type of initiator used. These types are cationic polymerization, anionic polymerization, and free radical polymerization. Figure 3 shows the mechanism of chain-growth polymerization.
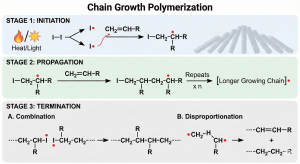
Figure 3: Chain-growth polymerization mechanism; created by Nano Banana Pro in Gemini
Comparing chain-growth and step-growth polymers
Now that we understand each type of polymerization, let’s compare the polymers created from the two different reactions, so step-growth and chain-growth polymers can be easily identified.
In step-growth polymerization, molecules of all sizes react together, such as dimers, trimers, and oligomers. In chain-growth polymerization, only active sites react, so monomers can only be added to the end of a growing chain. Step-growth polymerization involves multifunctional monomers, meaning the monomers has two or more reactive groups, which allows creation of oligomers. Chain-growth polymerization only contains a monomer that requires initiation. Step-growth does not require initiation or termination steps, while chain-growth does. Step-growth polymerization consumes monomer early in the reaction and high molecular weight polymerization is created at the end of a reaction, while monomers are consumed and molecular weight is increased consistently in chain-growth polymerization, which is shown in Figure 4 1.
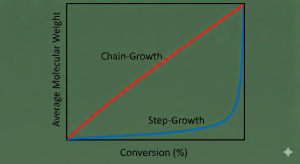
Figure 4: Graph showing average molecular weight vs. conversion % for Chain-Growth and Step-Growth polymerization; created by Nano Banana Pro in Gemini
Article Summary
Understanding Polymerization Terminology
The article “Reconsidering terms for mechanisms of polymer growth: the “step-growth” and “chain-growth” dilemma” by Chan et al. explores the use of terminology to describe polymerization types and how this can be improved 3. The terms chain and step growth are the most accurate terminology we use, but can be misleading as a chain and steps are in all types of polymerization. This review dives into the issues associated with these names, and calls attention the need for different terminology 3.
The original terminology created by Wallace Carothers in the 1930s was addition and condensation polymerization. He defined these as addition polymers as polymers that have the same repeat unit as the structure, and condensation polymers as polymers where the repeat unit differs from the monomer repeat unit, by way of the loss of a small molecule. However, there are cases where this definition leads to incorrect classification of polymers. The example stated in the article is polyurethane, which is technically made through step-growth (or condensation) polymerization, but there is no small molecule lost during the reaction. Carother’s definition would lead many to classify this reaction as addition polymerization, which would be incorrect 3.
The terms step and chain-growth polymerization are not perfect either. Chain-growth polymerization involves three steps, while step-growth polymerization does not, which can initiate confusion. These terms are considered vague to many, as both step and chain are components of every type of polymerization. IUPAC attempted to make more clear terminology and definitions for step-growth polymerization in 1994, with polycondensation and polyaddition. They defined polyaddition as “polymers formed by addition reactions between molecules of all sizes (“step-growth”), usually in a non-chain reaction, without low-molar-mass by-products” and polycondensation as “polymers formed through condensation reactions between molecules of all sizes (“step-growth”), usually in a non-chain reaction, producing low-molar-mass by-products”. However, it is difficult to get this terminology adopted universally, as it is ambiguous and can be confusing to students 3.
This lack of clear terminology for understanding fundamental polymerization concepts fosters confusion in learning. This ambiguity and lack of consistency is especially an issue when translating literature to different languages. While many agree chain-growth and step-growth are the more accurate terms, they are not perfect and improvement is needed 3.
This article shows the importance of clear terminology and understanding of key characteristics in polymerization. Since terminology cannot be relied on for a clear understanding, polymerization types must be understood indepth, so students can differentiate between types for various polymers. Understanding the types of polymerization that is occurring is fundamental to polymer science.
Check your Understanding!
References
- Saldívar‐Guerra E, Vivaldo‐Lima E. Introduction to polymers and polymer types. Polymer Science, Engineering, and Sustainability. 2025 Oct 28;1:1-8.
- Ribeiro AP, Martins MO, Figueiras AO, Martins LM. Introduction to Polymerization and Depolymerization. InDepolymerization: Concepts, Progress, and Challenges Volume 1: Core Concepts and Fundamentals 2025 (pp. 1-23). American Chemical Society.
- Chan CH, Chen JT, Farrell WS, Fellows CM, Keddie DJ, Luscombe CK, Matson JB, Merna J, Moad G, Russell GT, Théato P. Reconsidering terms for mechanisms of polymer growth: the “step-growth” and “chain-growth” dilemma. Polymer Chemistry. 2022;13(16):2262-70.