15 Structure-Property Relationships in Polymeric Fluorescent Probes: Design Principles and Applications
Rony Mia
1. Introduction:
Fluorescence, a captivating phenomenon where molecules absorb light and re-emit it at longer wavelengths, has become an indispensable tool in modern science and technology. At the forefront of this field are polymeric fluorescent probes, sophisticated macromolecular structures that combine the versatility of polymers with the sensitivity of fluorescence detection. These probes have revolutionized numerous applications, from biological imaging and environmental sensing to security measures and optoelectronic devices [1].
The allure of polymeric fluorescent probes lies in their remarkable ability to translate molecular-level interactions into observable optical signals. Unlike small molecule fluorophores, polymeric probes offer enhanced sensitivity, improved photostability, and the potential for multifunctional capabilities. The polymer backbone serves not merely as a passive scaffold but as an active participant in the probe’s performance, influencing everything from solubility and aggregation behavior to the very nature of the fluorescence emission [2].
At the heart of designing effective polymeric fluorescent probes is a deep understanding of structure-property relationships. The intricate interplay between chemical composition, molecular architecture, and macroscopic properties determines the probe’s fluorescence characteristics, sensitivity to target analytes, and overall performance in various applications. By manipulating these structural elements, scientists can fine-tune probe properties such as emission wavelength, quantum yield, and response to environmental stimuli [3].
This chapter aims to unravel the complex world of structure-property relationships in polymeric fluorescent probes. We will explore the fundamental principles that govern fluorescence in polymeric systems, examine key design strategies for enhancing probe performance, and investigate the synthesis and characterization techniques crucial to their development. Through this journey, we will see how seemingly minor alterations in molecular structure can lead to profound changes in a probe’s behavior and functionality.
As we introduce into the applications of these remarkable materials, from tracking cellular processes to detecting environmental pollutants, we will witness the far-reaching impact of polymeric fluorescent probes across diverse fields. The interdisciplinary nature of this topic underscores the importance of bridging chemistry, physics, and materials science in addressing complex real-world challenges.
By the end of this chapter, readers will gain not only a theoretical understanding of how molecular structure influences fluorescence properties in polymeric systems but also practical insights into designing and applying these probes. As we stand on the cusp of new discoveries and innovations in this dynamic field, the principles discussed here will serve as a foundation for future advancements, inspiring the next generation of scientists and engineers to push the boundaries of what’s possible with polymeric fluorescent probes.
2. Fundamentals
2.1. Basic Principles of Fluorescence
Fluorescence is a luminescence phenomenon where a substance absorbs light at one wavelength and emits light at a longer wavelength [4]. This process involves several key concepts:
- Excitation: Absorption of a photon promotes an electron from the ground state to an excited state.
- Vibrational relaxation: The excited electron rapidly loses energy through non-radiative processes, settling to the lowest vibrational level of the excited state.
- Emission: The electron returns to the ground state, releasing a photon of lower energy (longer wavelength) than the absorbed photon.
- Stokes shift: The difference between the excitation and emission wavelengths, typically ranging from 10 to 100 nm.
- Quantum yield: The ratio of emitted to absorbed photons, indicating the efficiency of the fluorescence process.
- Fluorescence lifetime: The average time a fluorophore remains in its excited state before emitting a photon.
- Quenching: Processes that decrease fluorescence intensity, such as collisional quenching or Förster Resonance Energy Transfer (FRET).
2.2. Key Polymer Concepts
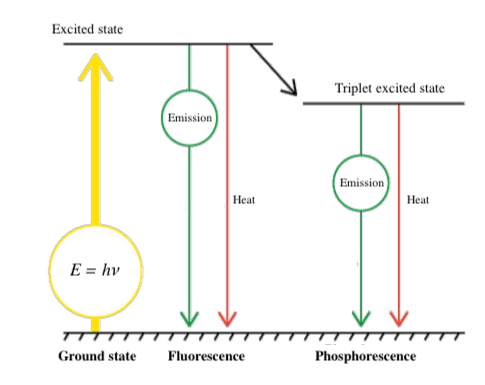
To fully grasp the structure-property relationships in polymeric fluorescent probes, it’s essential to understand fundamental polymer concepts (Figure 1):
- Polymer composition: The chemical nature of the repeating units that make up the polymer chain, which can significantly influence the probe’s properties.
- Polymer structure: The arrangement of monomers in the chain, including linear, branched, or network structures, affecting the probe’s physical and optical characteristics.
- Molecular weight: The sum of the molecular weights of all repeating units, impacting properties like solubility, viscosity, and chain dynamics.
- Polydispersity: The distribution of molecular weights in a polymer sample, which can affect the uniformity of the probe’s properties.
- Conformation: The spatial arrangement of the polymer chain, influenced by factors such as solvent interactions and temperature.
- Chain dynamics: The motion and flexibility of polymer chains, which can impact the probe’s response to environmental changes.
- Copolymerization: The incorporation of two or more different monomers in a single polymer chain, allowing for fine-tuning of properties.
- Functional groups: Chemical moieties along the polymer backbone or as side groups that can influence fluorescence properties and enable further modifications.
Figure 1: Jablonski diagram illustrating the absorption, vibrational relaxation, and emission processes in fluorescence.
3. Structure-Property Relationships
3.1. Chemical Structure and Fluorescence Properties
The chemical structure of polymeric fluorescent probes plays a crucial role in determining their fluorescence properties:
- Conjugated systems: Extended π-conjugation in the polymer backbone or side chains typically leads to red-shifted absorption and emission spectra, and can enhance fluorescence intensity (Figure 2).
- Electron-donating and electron-withdrawing groups: These can modulate the HOMO-LUMO energy gap, affecting the probe’s absorption and emission wavelengths. Electron-donating groups often increase fluorescence quantum yield, while electron-withdrawing groups can enhance sensitivity to specific analytes.
- Heteroatoms: Incorporation of heteroatoms (e.g., N, O, S) can influence the electronic structure, leading to changes in fluorescence properties and enabling specific interactions with analytes.
- Functional groups: Presence of certain functional groups can enable stimuli-responsiveness (e.g., pH-sensitive groups) or provide sites for further modification (e.g., click chemistry).
- Rigidity: Rigid structures often exhibit higher quantum yields due to reduced non-radiative decay pathways.
- Intramolecular charge transfer (ICT): Incorporation of donor-acceptor systems can lead to ICT states, resulting in large Stokes shifts and environment-sensitive emission.
3.2. Polymer Architecture Effects
The architecture of polymeric fluorescent probes significantly influences their performance and properties [5]:
- Linear polymers: Offer simplicity in synthesis and characterization, but may suffer from self-quenching at high concentrations due to interchain interactions.
- Branched polymers: Can reduce self-quenching and improve solubility compared to linear counterparts. Hyperbranched polymers often exhibit enhanced sensitivity due to their globular structure.
- Block copolymers: Allow for the combination of different functionalities, such as a hydrophilic block for water solubility and a hydrophobic block containing fluorophores. They can self-assemble into nanostructures, enabling applications like fluorescent nanoparticles.
- Dendrimers: Highly branched, monodisperse structures that offer precise control over fluorophore placement and number. Their globular shape can protect core fluorophores from the environment.
- Brush polymers: Dense side chains can provide a protective environment for fluorophores, potentially enhancing quantum yield and reducing quenching.
- Cross-linked networks: Can create three-dimensional fluorescent hydrogels with unique swelling and optical properties, useful for sensing applications.
- Star polymers: Combine aspects of linear and branched architectures, offering opportunities for multifunctional probes.
Figure 2: Structure-Property Relationships
4. Synthesis and Characterization
4.1. Common Synthesis Methods
The synthesis of polymeric fluorescent probes involves various polymerization techniques and post-polymerization modifications:
- Controlled Radical Polymerization (CRP):
- Atom Transfer Radical Polymerization (ATRP)
- Reversible Addition-Fragmentation Chain Transfer (RAFT)
- Nitroxide-Mediated Polymerization (NMP)
These methods offer control over molecular weight, polydispersity, and architecture, enabling the synthesis of well-defined fluorescent polymers.
- Click Chemistry:
Highly efficient reactions like copper-catalyzed azide-alkyne cycloaddition (CuAAC) or thiol-ene click reactions allow for facile incorporation of fluorophores into polymer backbones or as pendant groups. - Ring-Opening Polymerization (ROP):
Useful for synthesizing biodegradable fluorescent polymers, such as those based on lactide or caprolactone monomers. - Condensation Polymerization:
Enables the synthesis of conjugated polymers with intrinsic fluorescence, such as poly(p-phenylene vinylene) derivatives. - Post-polymerization Modification:
Allows for the attachment of fluorophores to pre-formed polymers, offering flexibility in probe design and the ability to use sensitive fluorophores that might not withstand polymerization conditions [6]. - Supramolecular Assembly:
Non-covalent interactions can be used to create fluorescent polymeric structures, such as self-assembled block copolymer micelles.
4.2. Key Characterization Techniques:
Accurate characterization of polymeric fluorescent probes is crucial for understanding their structure-property relationships:
- Spectroscopic Methods:
- UV-Visible Spectroscopy: Determines absorption characteristics
- Fluorescence Spectroscopy: Measures emission spectra, quantum yields, and fluorescence lifetimes
- Circular Dichroism: Analyzes chiral structures and conformational changes
- Molecular Weight Determination:
- Gel Permeation Chromatography (GPC): Provides molecular weight distribution
- Light Scattering: Offers absolute molecular weight measurements
- Nuclear Magnetic Resonance (NMR) Spectroscopy:
Elucidates chemical structure and can provide information on polymer tacticity and sequence distribution in copolymers - Microscopy Techniques:
- Transmission Electron Microscopy (TEM): Visualizes nanostructures
- Atomic Force Microscopy (AFM): Examines surface morphology and mechanical properties
- Thermal Analysis:
- Differential Scanning Calorimetry (DSC): Determines thermal transitions
- Thermogravimetric Analysis (TGA): Assesses thermal stability
- Dynamic Light Scattering (DLS):
Measures particle size and distribution in solution - X-ray Techniques:
- X-ray Diffraction (XRD): Analyzes crystalline structures
- Small-Angle X-ray Scattering (SAXS): Probes nanoscale structures
These techniques provide comprehensive information about the physical, chemical, and optical properties of polymeric fluorescent probes, guiding their development and application.
5. Applications
5.1. Biological Imaging and Sensing
Polymeric fluorescent probes have become invaluable tools in biological imaging and sensing due to their enhanced sensitivity [7], biocompatibility, and versatility:
- Cellular Imaging:
- Polymeric probes can be designed to target specific cellular components, such as membranes, organelles, or nucleic acids, providing detailed visualization of cellular processes.
- Their ability to incorporate multiple fluorophores allows for multiplexed imaging, enabling simultaneous observation of different targets.
- Biosensing:
- These probes can detect biomolecules like proteins, nucleic acids, and small metabolites with high specificity and sensitivity.
- They are often used in fluorescence resonance energy transfer (FRET)-based assays to monitor molecular interactions in real time.
- Drug Delivery Monitoring:
- Fluorescent polymers can be integrated into drug delivery systems to track the release and distribution of therapeutics within the body.
- This capability aids in optimizing drug delivery strategies and assessing therapeutic efficacy.
- Advantages in Biological Systems:
- Polymeric probes often exhibit reduced cytotoxicity compared to small molecule dyes.
- Their tunable properties allow for customization to suit specific biological environments, enhancing their utility in complex biological systems.
5.2. Environmental and Industrial Uses
Polymeric fluorescent probes also find significant applications in environmental monitoring and industrial processes:
- Water Quality Monitoring:
- These probes can detect pollutants such as heavy metals, organic compounds, and pathogens in water sources.
- They offer rapid, on-site analysis capabilities, crucial for maintaining safe water supplies.
- Chemical Process Control:
- In industrial settings, polymeric fluorescent probes are used to monitor chemical reactions and process parameters.
- Their sensitivity to changes in temperature, pH, or chemical composition allows for precise process optimization.
- Security and Anti-counterfeiting:
- Fluorescent polymers are employed in security inks and coatings for anti-counterfeiting measures on currency, documents, and products.
- Their unique fluorescence signatures make them difficult to replicate, providing a robust layer of protection.
- Environmental Sensing:
- These probes can detect environmental changes such as pH shifts or the presence of specific gases.
- They are used in smart packaging technologies that indicate product freshness or spoilage.
The versatility of polymeric fluorescent probes makes them essential tools across diverse fields, from healthcare to environmental science and industrial applications [8]. Their ability to provide real-time, sensitive detection continues to drive innovation and expand their range of uses.
6. Peer-reviewed paper
6.1 Brief overview:
6.2 Relation to the chapter:
This paper is highly relevant to our chapter “Structure-Property Relationships in Polymeric Fluorescent Probes: Design Principles and Applications” in several ways:
- Fluorescent probe design: The study exemplifies the rational design of polymeric fluorescent probes, incorporating AIE-active groups to enhance sensitivity. This aligns with our chapter’s focus on design principles for effective probes.
- Structure-property relationships: The paper extensively discusses how the chemical structure and pore architecture of the polymers influence their sensing properties. This directly relates to our chapter’s core theme of structure-property relationships in polymeric fluorescent probes.
- Characterization techniques: The researchers employ various characterization methods (e.g., FE-SEM, HR-TEM, N2 adsorption) to analyze the polymers’ structures. These techniques are likely covered in our chapter’s section on characterization methods.
- Application in pesticide detection: The paper demonstrates a practical application of polymeric fluorescent probes in environmental monitoring, specifically for pesticide detection. This case study could serve as an excellent example in our chapter’s application section.
- Photophysical mechanisms: The authors discuss photoinduced electron transfer (PET) and inner filter effect (IFE) mechanisms, which are crucial concepts in understanding the functioning of fluorescent probes. These mechanisms would likely be explained in our chapter’s fundamentals section.
- Performance metrics: The paper uses Stern-Volmer coefficients and limits of detection to quantify sensing performance. These metrics are important for evaluating fluorescent probes and would be covered in our chapter.
- Selectivity and interference studies: The researchers investigate the probes’ selectivity among different pesticides and their performance in the presence of interfering ions. This aspect is crucial for real-world applications and would be addressed in our chapter’s design principles section.
By incorporating insights from this paper, our chapter can provide up-to-date examples of how structure-property relationships are leveraged in the design of advanced polymeric fluorescent probes for specific applications like pesticide detection [10].
Paper link: https://doi.org/10.1021/acs.chemmater.2c02867
7. Conclusion:
Refereneces:
[2] Shen, R.; Liu, Y.; Yang, W. Y.; Hou, Y. Q.; Zhao, X. L.; Liu, H. Z. Triphenylamine-Functionalized Silsesquioxane-Based Hybrid Porous Polymers: Tunable Porosity and Luminescence for Multianalyte Detection. Chem. − Eur. J. 2017, 23, 13465−13472.
[3] Das, G.; Biswal, B. P.; Kandambeth, S.; Venkatesh, V.; Kaur, G.; Addicoat, M.; Heine, T.; Verma, S.; Banerjee, R. Chemical Sensing in Two Dimensional Porous Covalent Organic Nanosheets. Chem. Sci. 2015, 6, 3931−3939.
[4] Skorjanc, T.; Shetty, D.; Valant, M. Covalent Organic Polymers and Frameworks for Fluorescence-Based Sensors. ACS Sens. 2021, 6, 1461−1481.
[5] Lv, Z.; Chen, Z. X.; Feng, S. Y.; Wang, D. X.; Liu, H. Z. A Sulfur-Containing Fluorescent Hybrid Porous Polymer for Selective Detection and Adsorption of Hg2+ Ions. Polym. Chem. 2022, 13, 2320−2330.
[7] Hu, R.; Leung, N. L. C.; Tang, B. Z. AIE Macromolecules: Syntheses, Structures and Functionalities. Chem. Soc. Rev. 2014, 43, 4494−4562.
[8] Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361−5388.
[9] Yan, X.; Cook, T. R.; Wang, P.; Huang, F.; Stang, P. J. Highly emissive platinum(II) metallacages. Nat. Chem. 2015, 7, 342−348.
[10] Yan, X.; Wang, M.; Cook, T. R.; Zhang, M.; Saha, M. L.; Zhou, Z.; Li, X.; Huang, F.; Stang, P. J. Light-Emitting Superstructures with Anion Effect: Coordination-Driven Self-Assembly of Pure Tetraphenylethylene Metallacycles and Metallacages. J. Am. Chem. Soc. 2016, 138, 4580−4588.