34 Effect of Doping on Conjugated Polymer
Yusuf Olanrewaju
Learning Objectives
Upon the completion of this unit, students will be able to
- Understand the Fundamentals of Conjugated Polymers
- Explain the Mechanisms of Doping
- Explain the Impact of Doping on Polymer Properties
- Apply Knowledge to Advanced Applications
1.0 Introduction
Conjugated polymers are an intriguing class of materials widely used in the design of flexible electronic devices. Examples include polyaniline, polypyrrole, and polythiophene, which are inherently semiconducting due to their π-conjugated electronic structure [1]. Their applications range from organic light-emitting diodes (OLEDs) and organic solar cells to thermoelectric devices and bioelectronics (Figure 1) [2]. However, their baseline conductivity is often insufficient for practical applications. Doping in organic semiconductors, including conjugated polymers and small molecules (Figure 2), plays an important role in advancing organic electronics, enabling these materials to achieve semiconducting or metallic properties[3].
Doping is the process of introducing charge carriers into these materials through chemical or electrochemical means, dramatically enhances their electrical conductivity. The concept of doping was first applied to conjugated polymers in the late 1970s when Shirakawa et al. discovered that the conductivity of trans-polyacetylene increased by several orders of magnitude upon exposure to iodine vapor [4]. This breakthrough demonstrated the potential of conjugated polymers as viable materials for electronic applications. Doping introduces either positive (holes) or negative (electrons) charges into the polymer, altering its electronic structure, oxidation state, and physical properties. These changes facilitate charge carrier mobility, transforming conjugated polymers from insulators or weak semiconductors into materials with metallic-like conductivity.
The unique characteristics of doping in conjugated polymers stem from their organic, flexible nature, which differs significantly from inorganic semiconductors. Unlike the lattice-based doping mechanisms in traditional semiconductors, conjugated polymers rely on charge transfer reactions, oxidative or reductive processes, and interactions between the dopant and π-conjugated systems. Hence this chapter delves into the fundamental aspects of doping in conjugated polymers, including the classification of dopants, doping techniques, the role of dopants in conduction, and how polymer properties influence doping, with particular emphasis on the structural characteristics of the polymers.
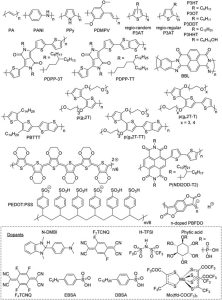
Figure 2: Chemical structures of the conjugated polymers and dopants[5].
2.0 Classification of Dopants for Conjugated Polymers
Dopants are chemical agents introduced into conjugated polymers to modulate their electronic properties. They are classified based on the type of charge carriers they generate when introduced into the polymer. The two main categories, p-type and n-type dopants, are analogous to doping in inorganic semiconductors like silicon, where boron acts as a p-type dopant and phosphorus as an n-type dopant. P-type dopants create positive charge carriers (holes) by removing electrons, while n-type dopants introduce negative charge carriers (electrons) into the polymer matrix.
P-type doping is achieved by adding oxidizing agents that readily accept electrons from the conjugated polymer backbone. This process leaves behind positive charges, often referred to as polarons, on the polymer chain. Common examples of p-type dopants include strong acids such as sulfuric acid and perchloric acid, organic acceptors such as 2,3,5,6-Tetrafluoro-7,7,8,8-tetracyanoquinodimethane (F4TCNQ), and metal salts like ferric chloride (FeCl3) [6]. These dopants play a critical role in improving the conductivity and charge transport properties of the polymer.
N-type doping involves the addition of reducing agents that donate electrons to the conjugated polymer, resulting in the formation of negative charge carriers. However, stabilizing n-type dopants is generally more challenging due to their sensitivity to environmental factors, particularly air. Examples of n-type dopants include lithium and sodium, as well as specialized organometallic compounds such as N-DMBI designed for enhanced stability and reactivity. The development of robust n-type dopants remains an area of active research in organic electronics. Dopants can also be classified based on their electron transfer mechanism, chemical nature, and doping process. These classifications provide a deeper understanding of how dopants interact with conjugated polymers and contribute to their electronic properties [7].
2.1 Classification by Electron Transfer
Dopants for conjugated polymers are often classified based on their ability to donate or accept electrons, leading to charge carrier generation. P-type dopants act as oxidizing agents, removing electrons from the polymer backbone and creating positive charges such as polarons or bipolarons. These dopants increase the hole conductivity of the material and are widely used in applications such as organic light-emitting diodes (OLEDs). Common examples include F4TCNQ, iodine, and FeCl3. In contrast, n-type dopants function as reducing agents, donating electrons to the polymer backbone to create negative charges, thereby enhancing electron conductivity. Sodium naphthalide and benzyl viologen are well-known examples of n-type dopants. These classifications reflect how dopants interact with the polymer electronic structure to modulate its properties, enabling tailored electronic performance for various applications [6].
2.2 Classification by Chemical Nature
Dopants can also be categorized by their chemical structure, ranging from small molecules to complex polymers. Small molecule dopants are low molecular weight compounds that intercalate into the polymer matrix, effectively tuning its conductivity. Examples include F4TCNQ, TCNQ, and iodine. Polymeric dopants are macromolecules that often enhance the stability and processability of the doped polymer. A common example is polystyrene sulfonate (PSS), used alongside polyaniline (PANI). Inorganic dopants, such as FeCl3 and H2SO4, are metallic or salt-based compounds that modify the electronic structure of polymers through strong oxidizing or protonating capabilities. Conversely, organic dopants include a range of compounds with functional groups or π-acceptor molecules, like TCNQ derivatives, which are tailored for specific interactions with conjugated polymers.
2.3 Classification by Doping Mechanism
The doping process can occur via different mechanisms that influence how charge carriers are introduced into the polymer. Chemical doping involves a direct chemical reaction between the dopant and the polymer, leading to the generation of charge carriers. For instance, FeCl3 oxidizes polythiophene, creating conductive sites. Electrochemical doping relies on applying an external voltage in the presence of an electrolyte, which facilitates the introduction of charge carriers into the polymer matrix. This method is commonly used for materials like polypyrrole. Photo-induced doping occurs when light excites the polymer, enabling charge transfer between the dopant and polymer. This technique is often applied in organic photovoltaic systems. Lastly, thermal doping uses high temperatures to allow dopants to diffuse into the polymer matrix, such as iodine sublimation doping, which is simple and effective for achieving uniform doping.
3.0 Doping Techniques for Conjugated Polymers
The choice of doping technique is critical for achieving the desired electronic and physical properties in conjugated polymers. The primary methods include chemical, electrochemical, and physical doping.
3.1 Chemical Doping
Chemical doping involves exposing the polymer to dopant vapors, solutions, or solid-phase dopants. The process typically relies on redox reactions between the polymer and the dopant. For instance, iodine vapor doping of trans-polyacetylene is a classic example of chemical p-type doping. Chemical doping is straightforward and scalable, making it suitable for large-scale production.
3.2 Electrochemical Doping
Electrochemical doping uses an applied potential to drive the doping process in a controlled manner. A polymer film is immersed in an electrolyte solution, and doping occurs at the anode (p-type) or cathode (n-type) during electrolysis. This technique allows precise control over the doping level and uniformity.
3.3 Physical Doping
Physical doping involves incorporating dopants into the polymer matrix during or after synthesis without chemical reactions. Techniques include co-polymerization, blending, or vapor deposition. Physical doping is often used for neutral or non-redox dopants.
4.0 Influence of Dopant on Polymer Structural and Electrical Properties
The efficiency and outcome of doping are profoundly influenced by the intrinsic properties of the conjugated polymer. The doping process in conjugated polymers is significantly influenced by their structural properties, including atomic connectivity, three-dimensional chain conformation, and crystalline arrangement. Morphology, which encompasses the polymer’s physical form, chain relationships, and aggregate structures, is closely tied to the polymer’s underlying structure. During doping, counter ions introduced to stabilize charges along the conjugated backbone can disrupt the rigid π-electron system, potentially causing structural distortions. These distortions may negatively impact the polymer’s crystallinity and morphology, ultimately affecting its performance. Highly ordered polymers exhibit better conductivity due to efficient charge transport. However a typical example shown in the figure 3 is the 2D grazing incidence wide-angle X-ray scattering (2D-GIWAXS) is used to study molecular packing in doped polymers. Pristine Pg32T-OTz exhibits a face-on orientation, transitioning to a bimodal orientation at a molar ratio (MR) of 0.25, and eventually to an edge-on orientation at MR > 0.4. In contrast, doped Pg32T-TT films consistently show edge-on orientations [8]. This indicates that the polymer backbone orientation relative to the substrate can be controlled through the solution doping process, potentially influencing the packing properties prior to film formation.
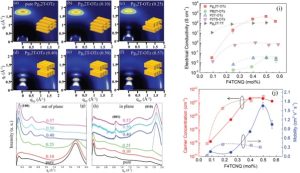
Figure 3: (a–f) 2D-GIWAXS scattering patterns of Pg32T-OTz with varied MR (insertions show the orientation of polymer chains in the film). (g) Out-of-plane line-cut profiles and (h) in-plane line-cut profiles with varied MR. (h) Electrical conductivity of all polymers with varied MR. (i) Carrier concentration and mobility of Pg32T-OTz (solid lines) and Pg32T-TT (dash lines) with varied F4TCNQ [8].
5.0 Effect of doping on Optical Properties
Conjugated polymer doped can significantly affects its optical properties by introducing new absorption bands in the visible and near-infrared spectrum due to the creation of charge carriers called polarons, which alter the electronic structure of the polymer, leading to changes in its color and overall light absorption behavior; essentially, doping enhances the polymer conductivity by generating free charge carriers, which also manifest in its optical properties. An example of these is the doping of P3HT and P(g32T-TT) that was observed with UV-vis/NIR spetra. Both polymers exhibited optical changes, including bleaching and the emergence of polaron/bipolaron bands (figure 4). For P3HT, doping led to the loss of the π–π* vibronic shoulder and a slight blue shift, attributed to polarons preferentially occupying more ordered regions of the polymer [9].
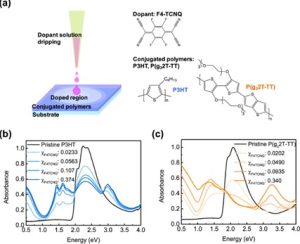
Figure 4: (a) Schematics of drip doping (sequential doping) of conjugated polymer films and the chemical structures of P3HT, P(g32T-TT), and F4TCNQ. UV–vis spectra of pristine and F4TCNQ-doped (b) P3HT and (c) P(g32T-TT) [9].
6.0 Conclusion
Doping in conjugated polymers is essential for unlocking their full potential in electronic and optoelectronic applications. This process, which introduces charge carriers and significantly enhances conductivity, has transformed polymers from insulators into materials with metallic-like properties suitable for use in a variety of devices, including OLEDs and organic solar cells. Understanding the different types of dopants (p-type and n-type) and their mechanisms, whether through chemical, electrochemical, or physical methods, allows for the precise modulation of polymer properties to meet specific application needs. The structural and optical effects of doping, such as changes in morphology and the introduction of new absorption bands, further highlight the importance of tailoring these properties for optimal performance. Through analytical techniques like UV-vis/NIR spectroscopy and 2D-GIWAXS, student can gain valuable insights into these transformations. This comprehensive understanding of doping not only aids in the development of more efficient and robust materials but also paves the way for future advancements in flexible, sustainable electronic technologies.
Interactive Videos:
References
[1] Z. Qiu, B.A.G. Hammer, K. Müllen, Conjugated polymers – Problems and promises, Prog. Polym. Sci. 100 (2020) 101179. https://doi.org/10.1016/J.PROGPOLYMSCI.2019.101179.
[2] K. Namsheer, C.S. Rout, Conducting polymers: a comprehensive review on recent advances in synthesis, properties and applications, RSC Adv. 11 (2021) 5659–5697. https://doi.org/10.1039/D0RA07800J.
[3] K. Pei, Recent Advances in Molecular Doping of Organic Semiconductors, Surfaces and Interfaces. 30 (2022) 101887. https://doi.org/10.1016/J.SURFIN.2022.101887.
[4] H. Shirakawa, E.J. Louis, A.G. MacDiarmid, C.K. Chiang, A.J. Heeger, Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x, J. Chem. Soc. Chem. Commun. (1977) 578–580. https://doi.org/10.1039/C39770000578.
[5] S.H.K. Paleti, Y. Kim, J. Kimpel, M. Craighero, S. Haraguchi, C. Müller, Impact of doping on the mechanical properties of conjugated polymers, Chem. Soc. Rev. 53 (2024) 1702–1729. https://doi.org/10.1039/D3CS00833A.
[6] D. Kiefer, R. Kroon, A.I. Hofmann, H. Sun, X. Liu, A. Giovannitti, D. Stegerer, A. Cano, J. Hynynen, L. Yu, Y. Zhang, D. Nai, T.F. Harrelson, M. Sommer, A.J. Moulé, M. Kemerink, S.R. Marder, I. McCulloch, M. Fahlman, S. Fabiano, C. Müller, Double doping of conjugated polymers with monomer molecular dopants, Nat. Mater. 2019 182. 18 (2019) 149–155. https://doi.org/10.1038/s41563-018-0263-6.
[7] Y.H. Li, J.Y. Wang, J. Pei, C-H σ-Dopants Mediated n-Doping of Conjugated Polymers: Mutual Designs and Multiscale Characteristics, Accounts Mater. Res. (2024). https://doi.org/10.1021/ACCOUNTSMR.4C00134/ASSET/IMAGES/LARGE/MR4C00134_0003.JPEG.
[8] S.J. Kwon, R. Giridharagopal, J. Neu, S. Kashani, S.E. Chen, R.J. Quezada, J. Guo, H. Ade, W. You, D.S. Ginger, Quantifying Doping Efficiency to Probe the Effects of Nanoscale Morphology and Solvent Swelling in Molecular Doping of Conjugated Polymers, J. Phys. Chem. C. 128 (2024) 2748–2758. https://doi.org/10.1021/ACS.JPCC.4C00153/SUPPL_FILE/JP4C00153_SI_001.PDF.
[9] H. Li, Z. Xu, J. Song, H. Chai, L. Wu, L. Chen, Single-Solution Doping Enabling Dominant Integer Charge Transfer for Synergistically Improved Carrier Concentration and Mobility in Donor–Acceptor Polymers, Adv. Funct. Mater. 32 (2022) 2110047. https://doi.org/10.1002/ADFM.202110047.
