26 Procedure
Preparation of Bacterial Samples (done by instructors)
- Grow isolates overnight by picking a colony from an agar plate and inoculating 2.5 mL of Tryptic Soy Broth (TSB). Grow at 30°C for 16-18 hours. Dilute in fresh medium and grow for 2-3 hours.
- We will prepare bacteria samples with concentrations around 107 cells/ml. Grow bacteria into late log phase in an appropriate medium. Note: according to the manufacturer, for E. coli culture, OD600 = 1.0 equals 8 x 108 cells/ml.
- Remove medium by centrifugation at 10,000 x g for 10 minutes and re-suspend the pellet in ddH2O, adjust bacteria concentration to ~ 107 cells/ml.
Staining Protocol
- Add 2 µL MycoLight™ dye working solution to 100 µL of the bacterial suspension.
- Mix well and incubate in the dark for 15 min at room temperature. You can store the solution in a 1.5 ml tube in your drawer.
- Remove the working solution by centrifugation at 10,000 g for 10 minutes.
- Resuspend the bacteria pellet in 100 µL volume of ddH2O.
- Add 10 µL of stained cells to a clean slide and cover with a coverslip. Provide the rest of the stained cells to your instructor for imaging.
- Monitor the fluorescence of bacteria with a fluorescent microscope or the Agilent LionHeart.
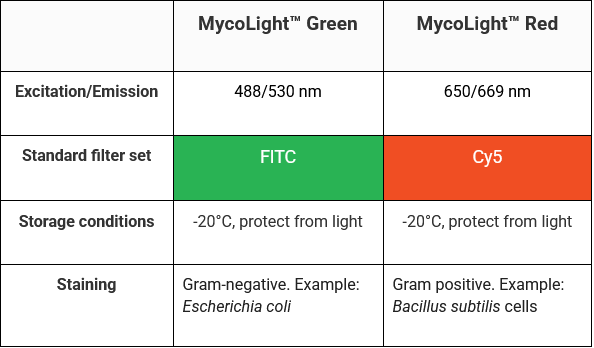
Table 2. MycoLight Bacterial Staining