9
Delftibactin Production
The gold precipitation gene in Delftia species is an adaptation that allows the bacterium to live in environments that contain gold in solution[1]. When gold is in a solution as gold ions, just like other heavy metals, it can be toxic and kill the bacterium[2]. However, solid gold is more stable and does not affect the bacteria. Because of its unique adaptation, Delftia can turn gold in solution into solid gold nanoparticles. The bacterium can even be found on gold nuggets as a biofilm!
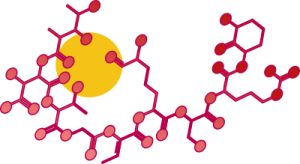
A cluster of genes named delA-delH encode for a complex called Delftibactin, which is what actually transforms the gold into solid nanoparticles[3]. Delftibactin is a secondary metabolite, meaning that it is not needed for day to day cell growth and function. The bacterium produces Delftibactin and then releases it into the environment, where it is able to reduce gold to a less reactive state by binding to gold molecules and reducing them by adding electrons to it[4]. This leads to the formation of gold nanoparticles.
- Johnston, C. W., Skinnider, M. A., Wyatt, M. A., Li, X., Ranieri, M. R., Yang, L., ... & Magarvey, N. A. (2015). An automated Genomes-to-Natural Products platform (GNP) for the discovery of modular natural products. Nature Communications, 6, 8421. ↵
- Hong, C. E., Jo, S. H., Jo, I., Jeong, H., & Park, J. M. (2017). Draft genome sequence of the endophytic bacterium. Genome Announcements, 5(36). ↵
- Johnston, C. W., Skinnider, M. A., Wyatt, M. A., Li, X., Ranieri, M. R., Yang, L., ... & Magarvey, N. A. (2015). An automated Genomes-to-Natural Products platform (GNP) for the discovery of modular natural products. Nature communications, 6, 8421. ↵
- Gold recycling: Using delftibactin to recycle gold from electronic waste. (2013). iGem Team Heidelberg. ↵

