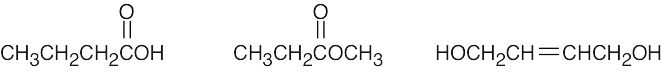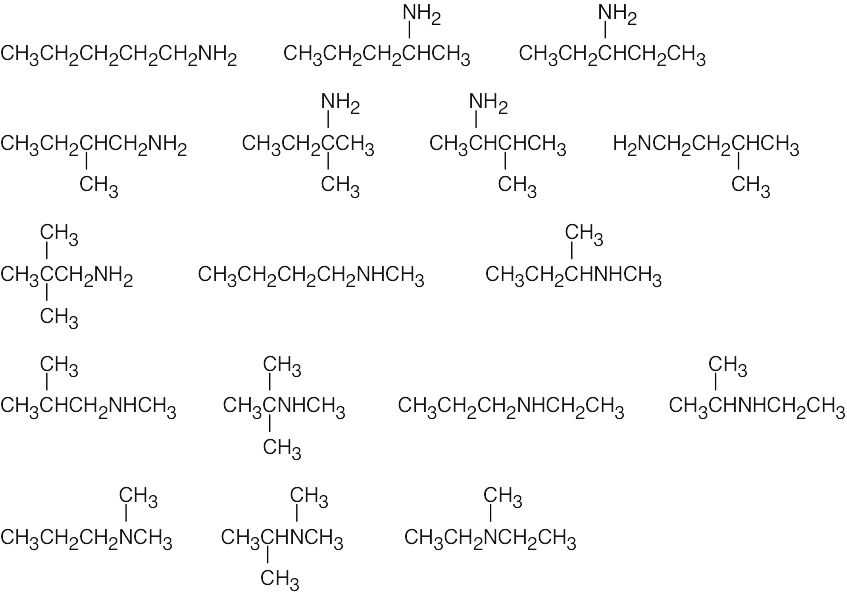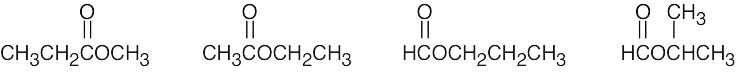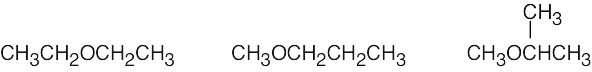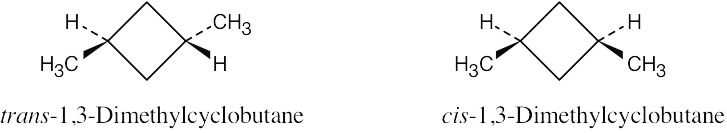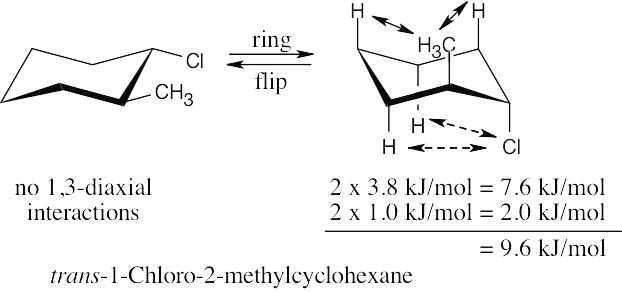2 Chapter 2 Solutions to Problems – Organic Compounds, Alkanes, Cycloalkanes and Their Stereochemistry
Chapter 2 – Organic Compounds, Alkanes, Cycloalkanes and Their Stereochemistry
Solutions to Problems
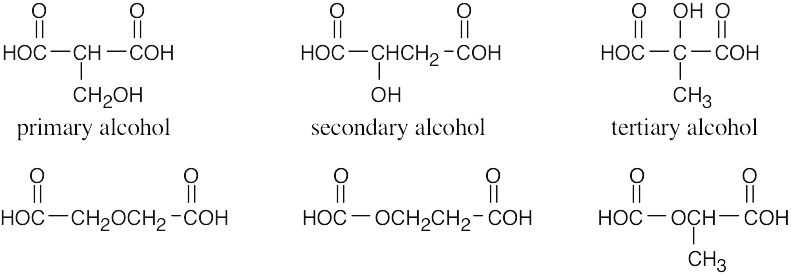
| 2.1 | Notice that certain functional groups have different designations if other functional groups are also present in a molecule. For example, a molecule containing a carbon–carbon double bond and no other functional group is an alkene; if other groups are present, the group is referred to as a carbon–carbon double bond. Similarly, a compound containing a benzene ring, and only carbon- and hydrogen-containing substituents, is an arene; if other groups are present, the ring is labeled an aromatic ring. | |
| (a) | 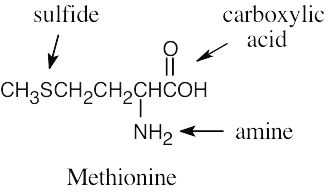 |
|
| (b) | 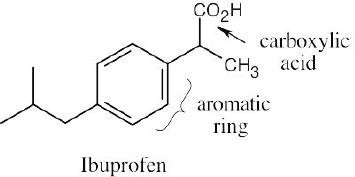 |
|
| (c) | 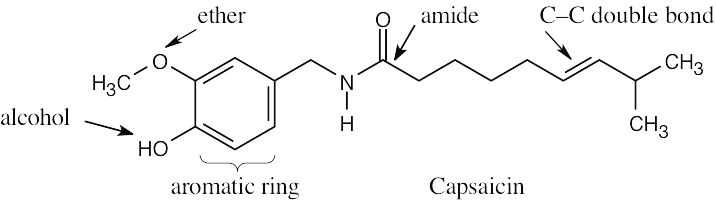 |
|
| 2.2 | (a) | 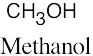 |
(d) | 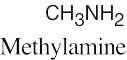 |
| (b) | 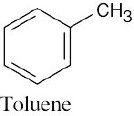 |
(e) | 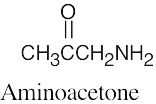 |
|
| (c) | 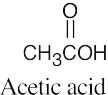 |
(f) | 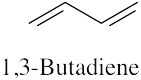 |
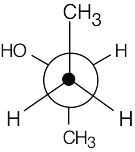
| 2.3 | 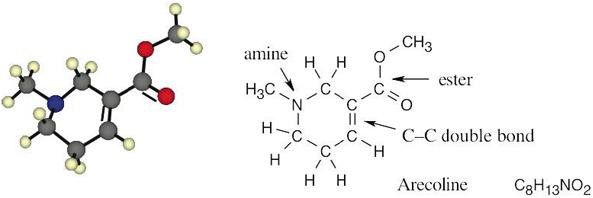 |
| 2.4 | We know that carbon forms four bonds and hydrogen forms one bond. Thus, draw all possible six-carbon skeletons and add hydrogens so that all carbons have four bonds. To draw all possible skeletons in this problem: (1) Draw the six-carbon straight-chain skeleton; (2) Draw a five-carbon chain, identify the different types of carbon atoms on the chain, and add a –CH3 group to each of the different types of carbons, generating two skeletons; (3) Repeat the process with the four-carbon chain to give rise to the last two skeletons. Add hydrogens to the remaining carbons to complete the structures.
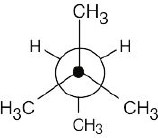
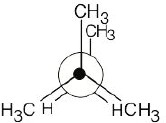
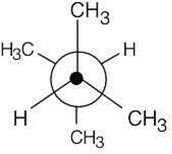
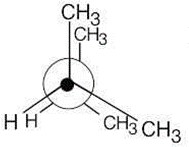
|
| 2.5 | (a) | Nine isomeric esters of formula C5H10O2 can be drawn. The procedure is described in Problem 3.4.
|
| (b) | Two isomers can be drawn.
|
| 2.6 | (a) | Two alcohols have the formula C3H8O.
|
| (b) | Four bromoalkanes have the formula C4H9Br.
|
|
| (c) | Four thioesters have the formula C4H8OS.
|
| 2.7 | 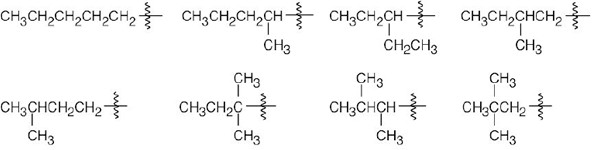 |
| 2.8 | (a) | 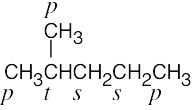 |
| (b) | 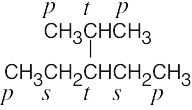 |
|
| (c) | 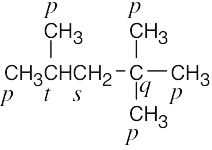 |
| 2.9 | The carbons and the attached hydrogens have the same classification. | |
| (a) | 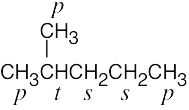 |
|
| (b) | 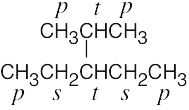 |
|
| (c) | 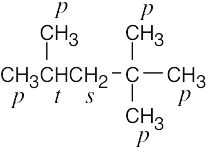 |
|
| 2.10 | (a) | 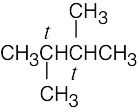 |
| (b) | 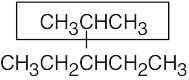 |
|
| (c) | 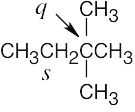 |
| 2.11 | (a) | 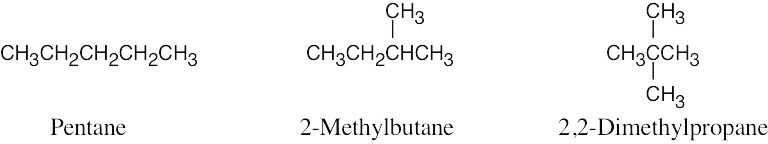 |
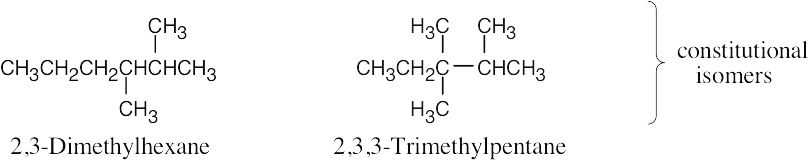
| (b) | Step 1: Find the longest continuous carbon chain and use it as the parent name. In (b), the chain is a pentane.
Step 2: Identify the substituents. In (b), both substituents are methyl groups. Step 3: Number the substituents. In (b), the methyl groups are in the 2- and 3- positions. Step 4: Name the compound. Remember that the prefix di– must be used when two are the same. The IUPAC name is 2,3-dimethylpentane.
|
|
| (c) | 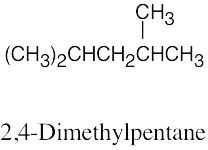 |
|
| (d) | 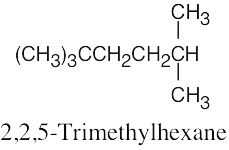 |
| 2.12 | When you are asked to draw the structure corresponding to a given name, draw the parent carbon chain, attach the specified groups to the proper carbons, and fill in the remaining hydrogens. | |
| (a) | 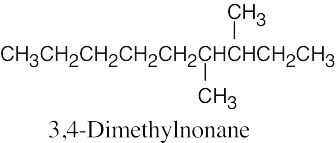 |
|
| (b) | 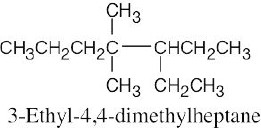 |
|
| (c) | 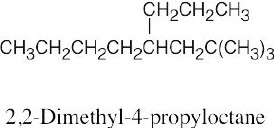 |
|
| (d) | 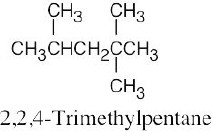 |
|
| 2.13 | 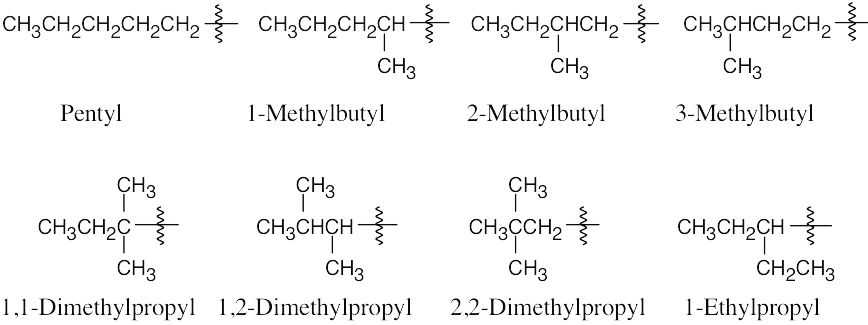 |
| 2.14 | 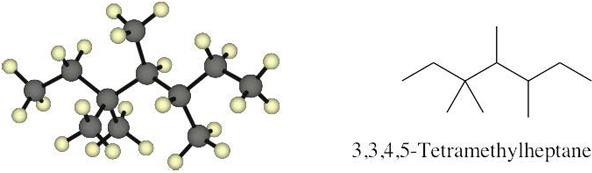 |
| 2.15 | The steps for naming a cycloalkane are very similar to the steps used for naming an open- chain alkane. | ||||||
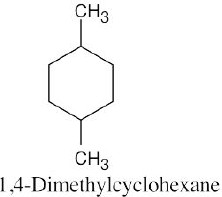
| Step 1: Name the parent cycloalkane. In (a), the parent is cyclohexane. If the compound has an alkyl substituent with more carbons than the ring size, the compound is named as a cycloalkyl-substituted alkane, as in (c).
Step 2: Identify the substituents. In (a), both substituents are methyl groups. Step 3: Number the substituents so that the second substituent receives the lowest possible number. In (a), the substituents are in the 1- and 4- positions. Step 4: Name the compound. If two different alkyl groups are present, cite them alphabetically. Halogen substituents follow the same rules as alkyl substituents.
|
| 2.16 | To draw a substituted cycloalkane, simply draw the ring and attach substituents in the specified positions. The structure in (b) is named as a cyclobutyl-substituted alkane because the alkyl chain has more carbons than the ring.
|
| 2.17 | |
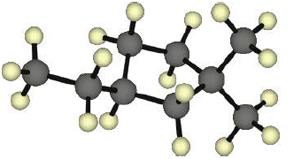 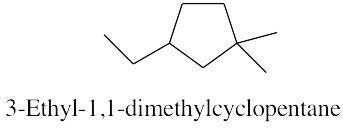 |
| 2.18 | (a) |  |
| (b) | 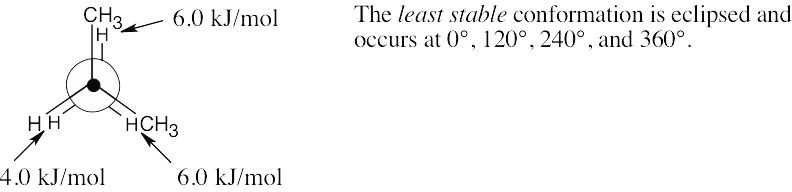 |
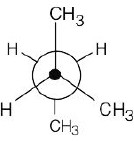
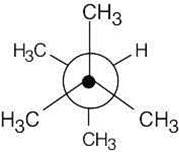
| 2.19 | This conformation of 2,3-dimethylbutane is the most stable because it is staggered and has the fewest CH3↔CH3 gauche interactions.
|
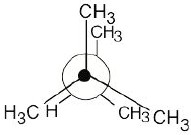
| 2.20 | The conformation is a staggered conformation in which the hydrogens on carbons 2 and 3 are 60° apart. Draw the Newman projection.
|
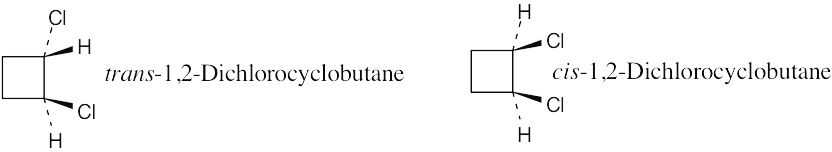
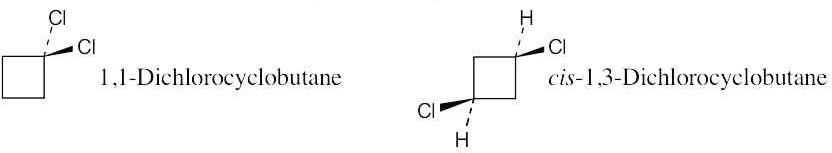
| 2.21 | Two substituents are cis if they both have either dashed or wedged bonds. The substituents are trans if one has a wedged bond and the other has a dashed bond.
|
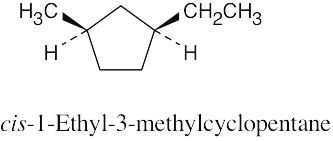
| 2.22 |
|
| 2.23 |
The two hydroxyl groups on the cyclopentyl ring are cis because they both point behind the plane of the page (both dashed bonds). The carbon chains have a trans relationship (one is dashed and the other is wedged). |
| 2.24 |
|
| 2.25 |
The added energy cost of eclipsing interactions causes cis-1,2-dimethylcyclopropane to be of higher energy and to be less stable than the trans isomer. |
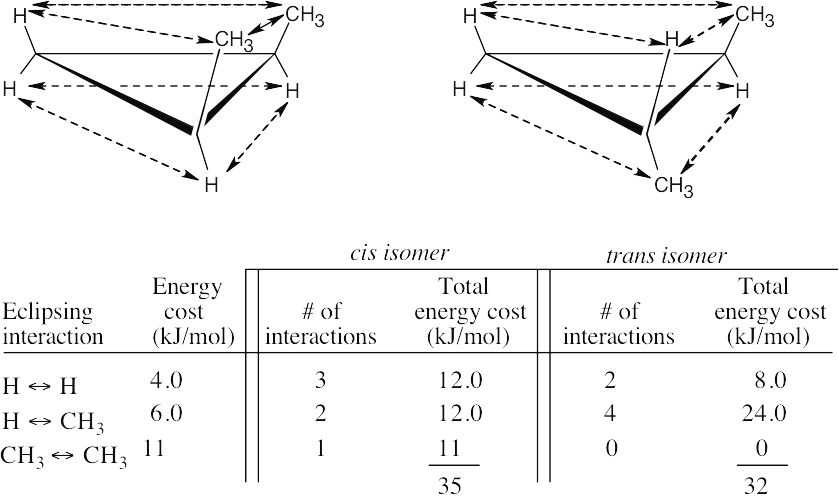
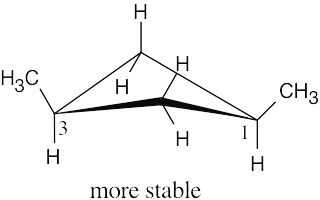
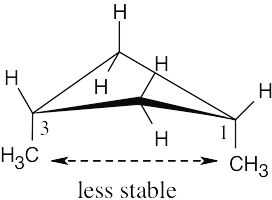
| 2.26 |
The methyl groups are farther apart in the more stable conformation of cis-1,3- dimethylcyclobutane. |
| 2.27 | Use the technique in Section 2.12 to draw the cyclohexane ring. Figure 2.12 shows how to attach axial (a) and equatorial (e) bonds.
The conformation with –OH in the equatorial position is more stable. Note: The starred ring carbons lie in the plane of the paper. |
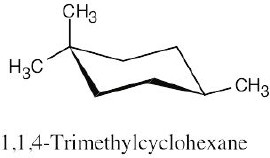
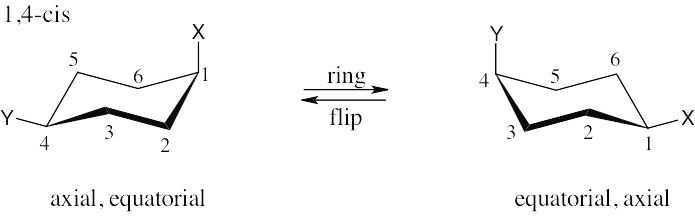
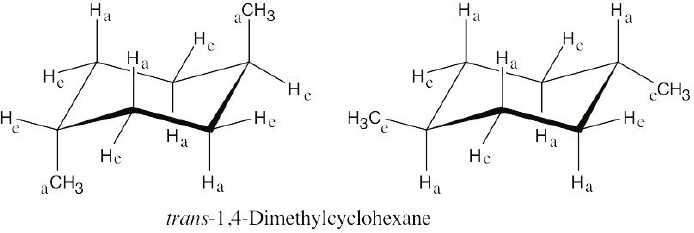
| 2.28 | In trans-1,4-disubstituted cyclohexanes, the methyl substituents are either both axial or both equatorial.
|
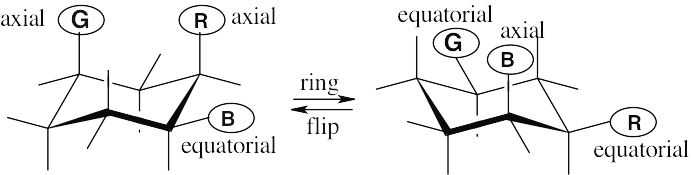
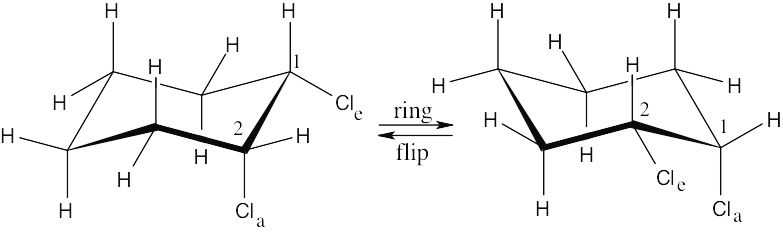
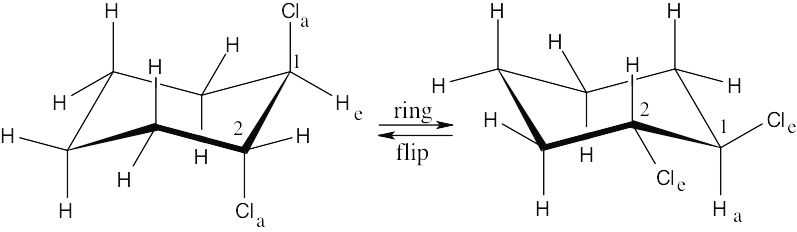
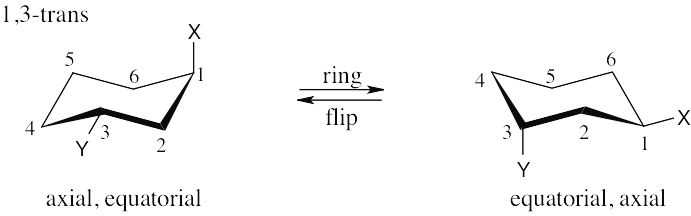
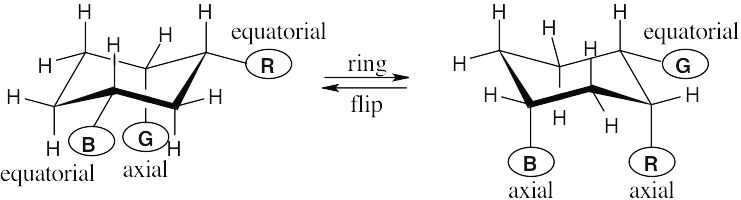
| 2.29 | In a ring-flip, an axial substituent becomes equatorial, and an equatorial substituent becomes axial.
|
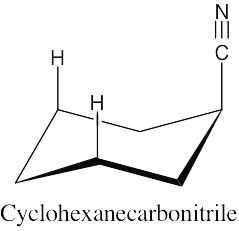
| 2.30 | There is very little energy difference between an axial and an equatorial cyano group because the small linear cyano group takes up very little room and produces practically no 1,3-diaxial interactions.
|
| 2.31 | The three substituents have the orientations shown in the first structure. To decide if the conformation shown is the more stable conformation or the less stable conformation, perform a ring-flip on the illustrated conformation and do a calculation of the total strain in each structure as in the previous problem. Notice that each conformation has a Cl – CH3 gauche interaction, but we don’t need to know its energy cost because it is present in both conformations.
The illustrated conformation on the left is the less stable chair form. |
Additional Problems
Visualizing Chemistry
| 2.32 | (a) | 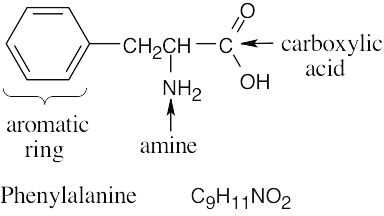 |
| (b) | 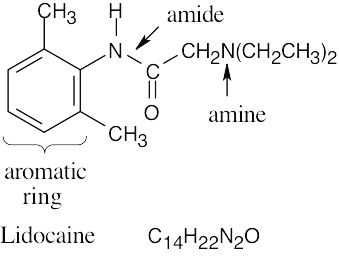 |
| 2.33 | (a) | 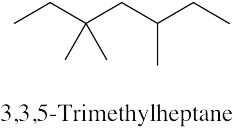 |
| (b) | 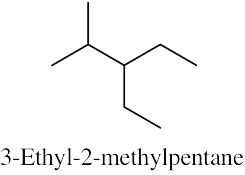 |
|
| (c) | 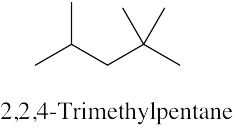 |
|
| (d) | 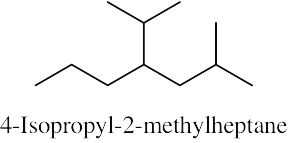 |
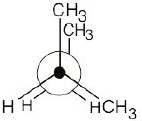
| 2.34 | In this conformation, all groups are staggered and the two methyl groups are 180° apart.
|
| 2.35 |
|
| 2.36 |
|
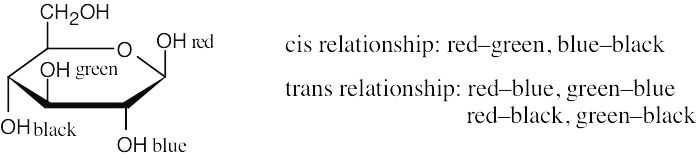
| 2.37 | The green substituent is axial, and the red and blue substituents are equatorial.
|
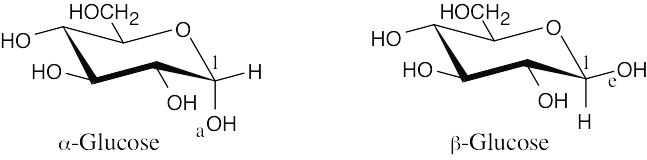
| 2.38 |
The only difference between α-glucose and β-glucose is in the orientation of the –OH group at carbon 1: the –OH group is axial in α-glucose, and it is equatorial in β-glucose. You would expect β-glucose to be more stable because all of its substituents are in the equatorial position. |
Functional Groups
| 2.39 | (a) | 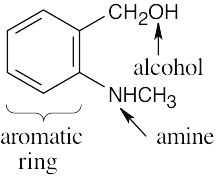 |
(d) | 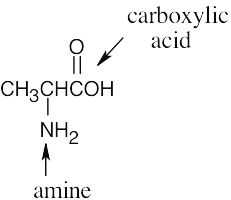 |
| (b) | 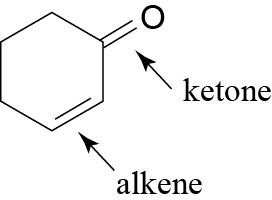 |
(e) | 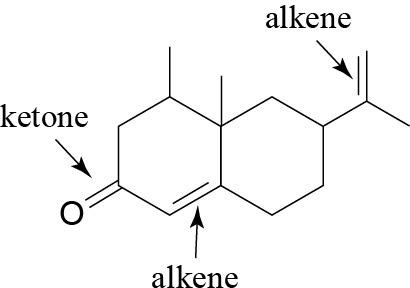 |
|
| (c) | 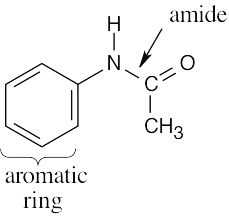 |
(f) | 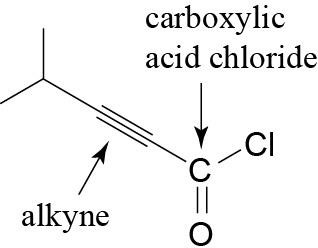 |
| 2.40 | Different answers to this problem and to Problem 3.24 are acceptable. | |||
| (a) | 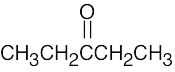 |
(d) | 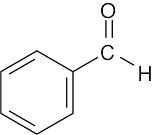 |
|
| (b) | 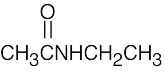 |
(e) | 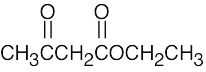 |
|
| (c) | 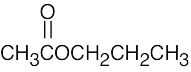 |
(f) | 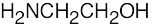 |
|
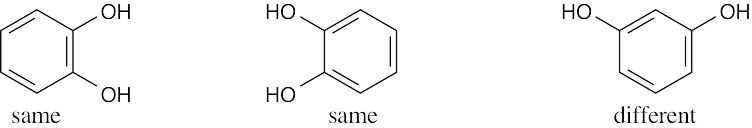
| 2.41 | For (a) and (h), only one structure is possible. | |||
| (a) | 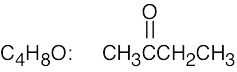 |
(e) | 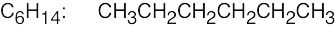 |
|
| (b) | 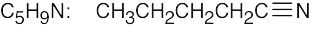 |
(f) | 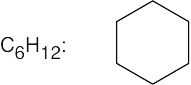 |
|
| (c) | 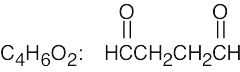 |
(g) | 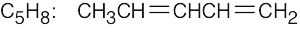 |
|
| (d) | 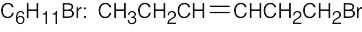 |
(h) | 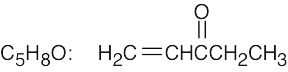 |
|
| 2.42 | (a) | 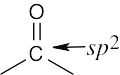 |
| (b) | 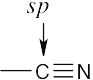 |
|
| (c) | 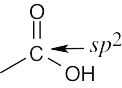 |
| 2.43 | (a) | Although it is stated that biacetyl contains no rings or carbon–carbon double bonds, it is obvious from the formula for biacetyl that some sort of multiple bond must be present. The structure for biacetyl contains two carbon–oxygen double bonds.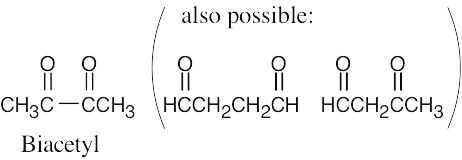 |
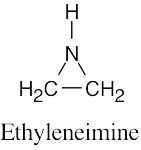
| (b) | Ethyleneimine contains a three-membered ring.
|
|
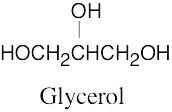
| (c) | Glycerol contains no multiple bonds or rings.
|
Isomers
| 2.44 | (a) | Eighteen isomers have the formula C8H18. Three are pictured.
|
| (b) | Structures with the formula C4H8O2 may represent esters, carboxylic acids or many other complicated molecules. Three possibilities:
|
| 2.45 | 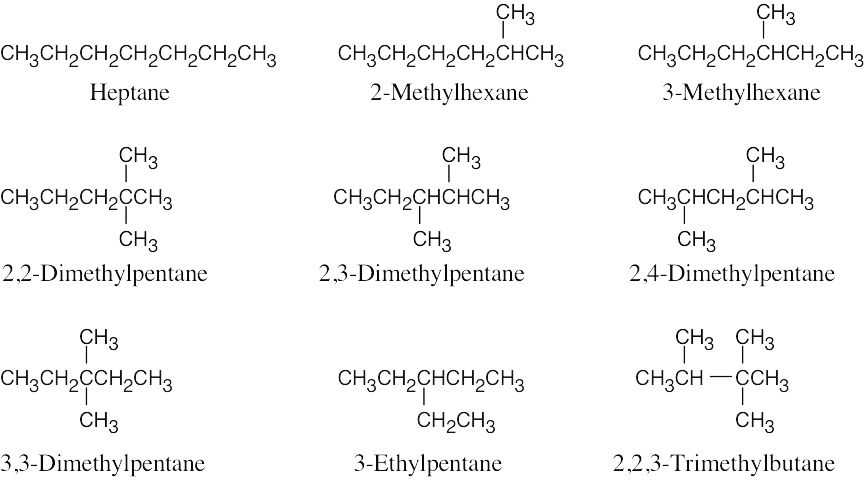 |
| 2.46 | (a) | 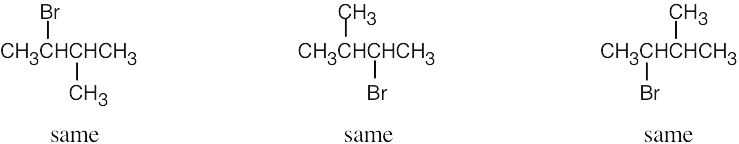 |
| (b) | Give the number “1” to the carbon bonded to –OH, and count to find the longest chain containing the –OH group.
|
|
| (c) | 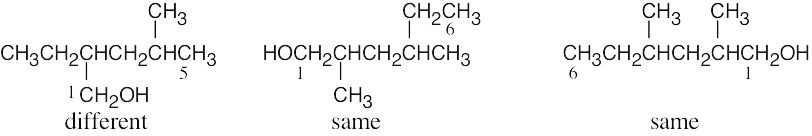 |
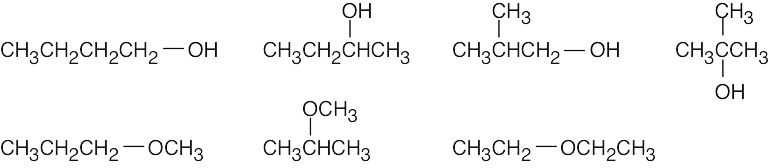
| 2.47 | The isomers may be either alcohols or ethers.
|
| 2.48 | First, draw all straight-chain isomers. Then proceed to the simplest branched structure. | |
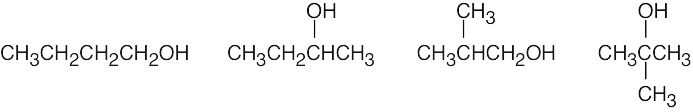
| (a) | There are four alcohol isomers with the formula C4H10O.
|
|
| (b) | There are 17 isomers of C5H13N. Nitrogen can be bonded to one, two or three alkyl groups.
|
|
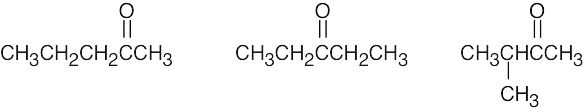
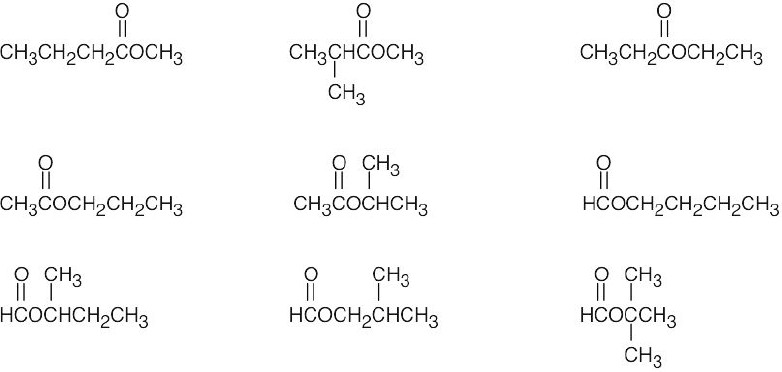
| (c) | There are 3 ketone isomers with the formula C5H10O.
|
|
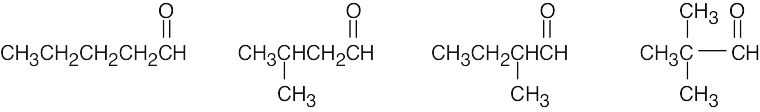
| (d) | There are 4 isomeric aldehydes with the formula C5H10O. Remember that the aldehyde functional group can occur only at the end of a chain.
|
|
| (e) | There are 4 esters with the formula C4H8O2.
|
|
| (f) | There are 3 ethers with the formula C4H10O.
|
|
| 2.49 | (a) |  |
(d) |  |
| (b) |  |
(e) | 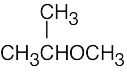 |
|
| (c) | 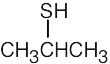 |
(f) | 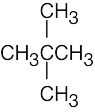 |
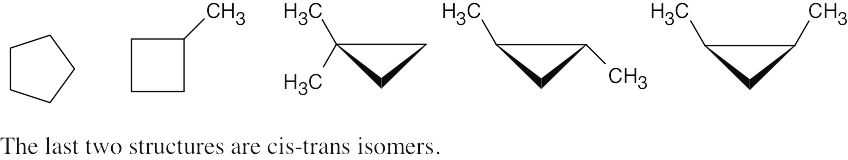
Cycloalkane Isomers
| 2.50 |
|
| 2.51 |
|
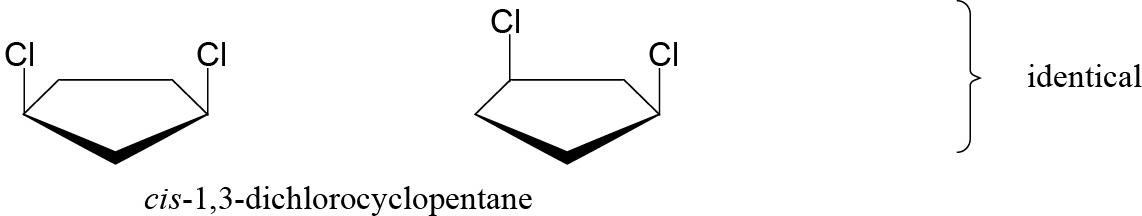
| 2.52 | Stereoisomers have different three-dimensional geometry.
|
| 2.53 |
|
| 2.54 | Stereoisomers:
|
| 2.55 |
|
Naming Compounds
| 2.56 | 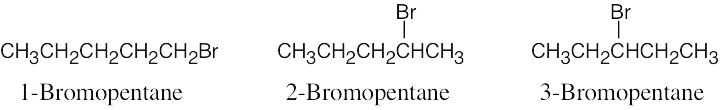 |
| 2.57 | 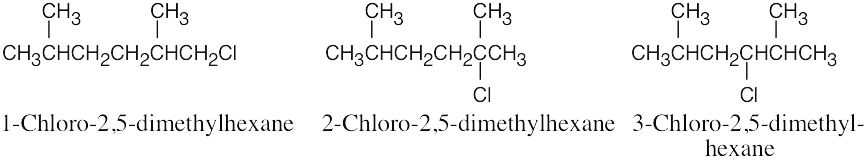 |
| 2.58 | (a) | 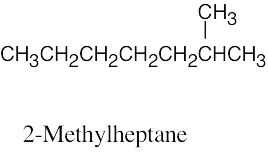 |
(d) | 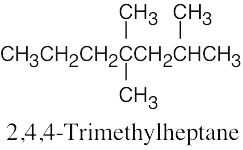 |
| (b) | 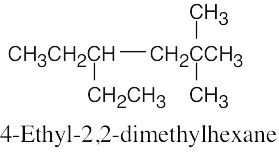 |
(e) | 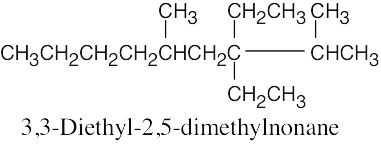 |
|
| (c) | 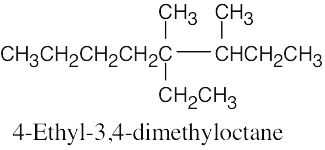 |
(f) | 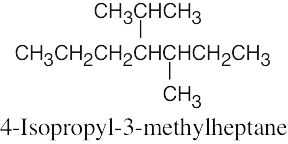 |
| 2.59 | (a) | 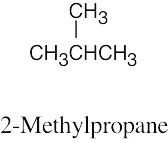 |
| (b) | 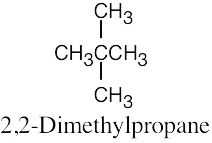 |
|
| (c) | 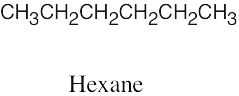 |
| 2.60 | (a) | 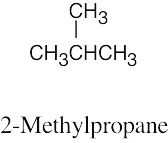 |
| (b) |  |
| 2.61 | (a) | 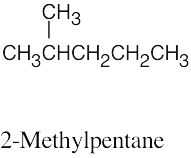 |
(d) | 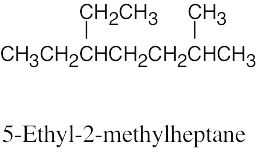 |
| (b) | 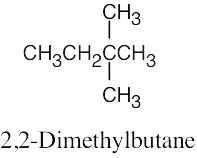 |
(e) | 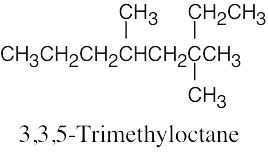 |
|
| (c) | 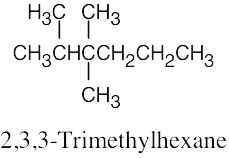 |
(f) | 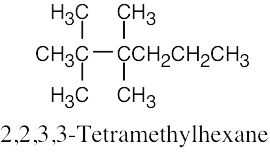 |
| 2.62 | 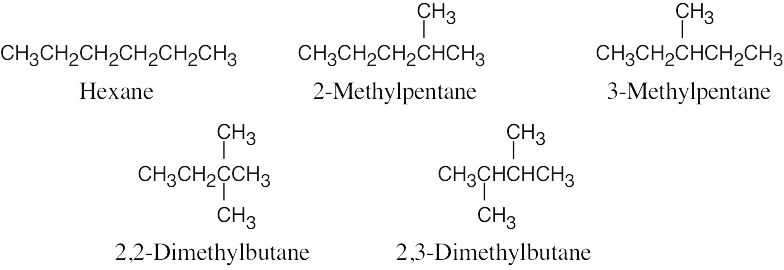 |
| 2.63 | Structure and Correct Name | Error | |
| (a) | 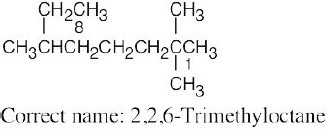 |
The longest chain is an octane and has only methyl branches. | |
| (b) | 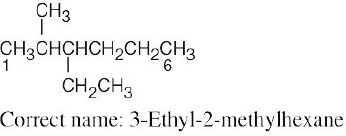 |
The longest chain is a hexane. Numbering should start from the opposite end of the carbon chain, nearer the first branch. | |
| (c) | 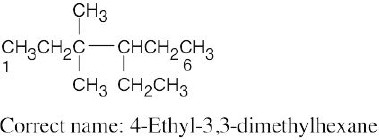 |
Numbering should start from the opposite end of the carbon chain. See step 2(b) in Section 2.4. | |
| (d) | 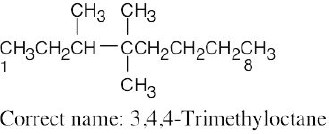 |
Numbering should start from the opposite end of the carbon chain. | |
| (e) | 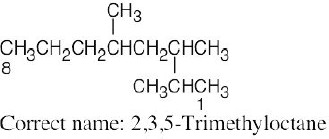 |
The longest chain is an octane. |
| 2.64 | (a) | 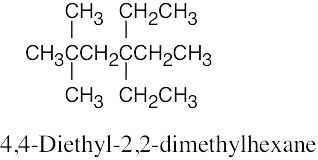 |
| (b) | 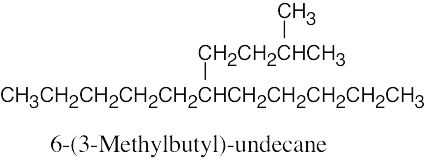
Remember that you must choose an alkane whose principal chain is long enough so that the substituent does not become part of the principal chain. |
Conformations
| 2.65 | (a), (b) | 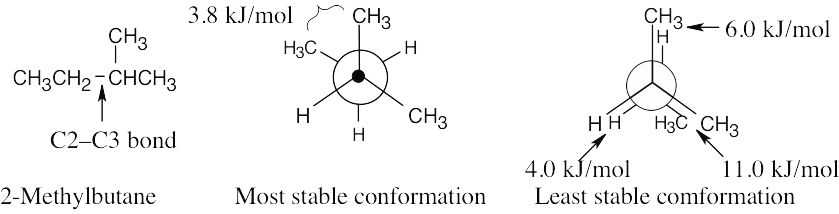
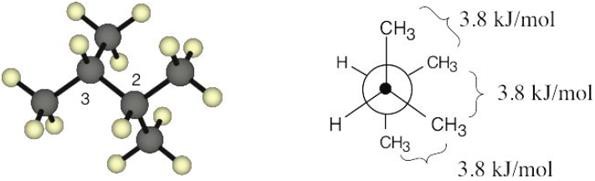
The energy difference between the two conformations is (11.0 + 6.0 + 4.0) kJ/mol – 3.8 kJ/mol = 17.2 kJ/mol. |
| 2.66 | The best way to draw pentane is to make a model and to copy it onto the page. A model shows the relationship among atoms, and its drawing shows these relationships in two dimensions. From your model, you should be able to see that all atoms are staggered in the drawing.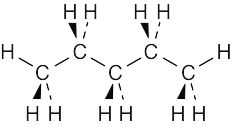 |
| 2.67 |  |
General Problems
| 2.68 | (a) | 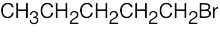 |
(d) | 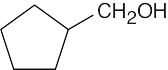 |
| (b) | 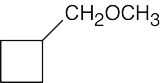 |
(e) | 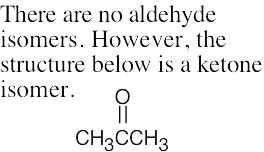 |
|
| (c) | 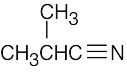 |
(f) | 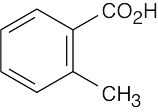 |
| 2.69 | (a) | Because malic acid has two –CO2H groups, the formula for the rest of the molecule is C2H4O. Possible structures for malic acid are:
|
| (b) | Because only one of these compounds (the second one) is also a secondary alcohol, it must be malic acid. |
| 2.70 |
|
Most stable |
Strain energy |
Least stable |
Strain energy |
|
(a) |
|
* 3.8 kJ/mol |
|
21 kJ/mol |
|
|
(b) |
|
7.6 kJ/mol |
|
23 kJ/mol |
|
|
(c) |
|
7.6 kJ/mol |
|
26 kJ/mol |
|
|
(d) |
|
15.2 kJ/mol |
|
** 28 kJ/mol |
|
|
|
*most stable overall (least strain)
**least stable overall (most strain) |
||||
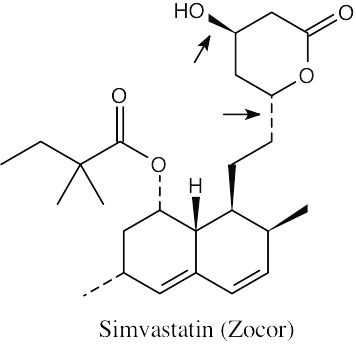
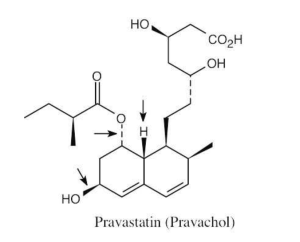
| 2.71 | The carboxylic acid and alcohol groups in Pravachol are an ester (lactone) group in Zocor. The alcohol at the bottom left of Pravachol is a methyl group in Zocor.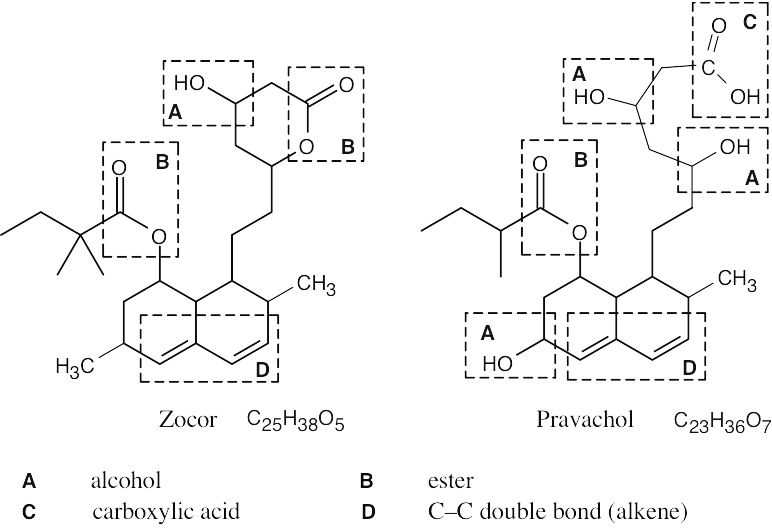 |
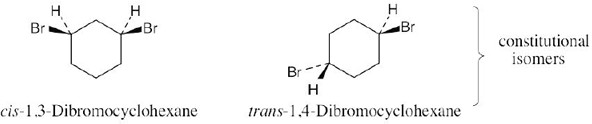
| 2.72 | In one of the 1,2-dimethylcyclohexanes the two methyl groups are on the same side of the ring, and in the other isomer the methyl groups are on opposite sides. |
| 2.73 |
(a) The indicated bonds on simvastatin are trans. (b) The –H bond and the –OH bond have a cis relationship. The third bond is trans to both of them. (c) The three indicated bonds on atorvastatin are attached to sp2-hybridized carbons of a planar ring and lie in the same plane. |
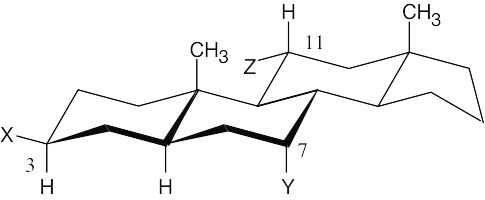
| 2.74 | A steroid ring system is fused, and ring-flips don’t occur. Thus, substituents such as the methyl groups shown remain axial. Substituents on the same side of the ring system as the methyl groups are in alternating axial and equatorial positions. Thus, an “up” substituent at C3 (a) is equatorial.
Substituents on the bottom side of the ring system also alternate axial and equatorial positions. A substituent at C7 (b) is axial, and one at C11 (c) is equatorial
|
Cycloalkane Conformation and Stability
| 2.75 | Make a model of cis-1,2-dichlorocyclohexane. Notice that all cis substituents are on the same side of the ring and that two adjacent cis substituents have an axial–equatorial relationship. Now, perform a ring-flip on the cyclohexane.
After the ring-flip, the relationship of the two substituents is still axial–equatorial. No two adjacent cis substituents can be converted to being both axial or both equatorial by a ring- flip. Don’t forget that there are only two chair conformations of any given cyclohexane. |
| 2.76 | For a trans-1,2-disubstituted cyclohexane, two adjacent substituents must be either both axial or both equatorial.
A ring flip converts two adjacent axial substituents to equatorial substituents, and vice versa. As in Problem 4.35, no two adjacent trans substituents can have an axial-equatorial relationship. |
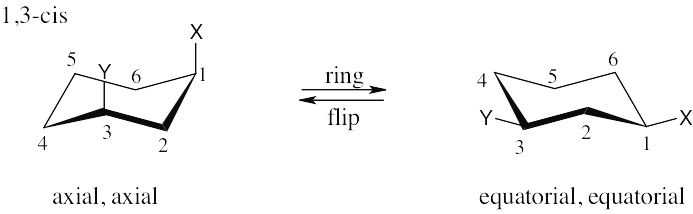
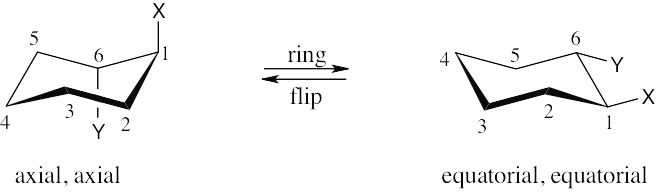
| 2.77 | Make sure you know the difference between axial–equatorial and cis–trans. Axial substituents are parallel to the axis of the ring; equatorial substituents lie around the equator of the ring. Cis substituents are on the same side of the ring; trans substituents are on opposite sides of the ring.
|
Cyclohexane Conformational Analysis
| 2.78 |
Use Table 4.1 to find the values of 1,3-diaxial interactions. The first conformation is more stable than the second conformation by a maximum of 9.6 kJ/mol. (A gauche interaction between the two substituents in the diequatorial conformation reduces the value of the energy difference, but its value can’t be determined with the given data.) |

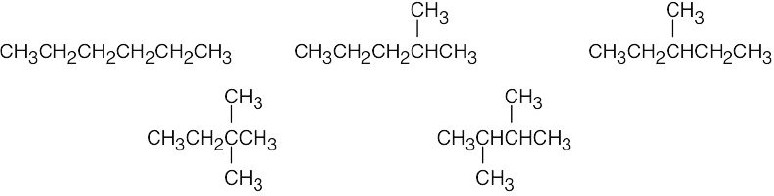
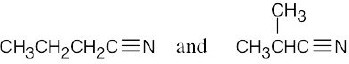
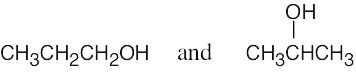
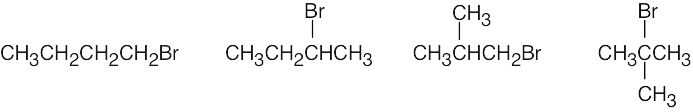

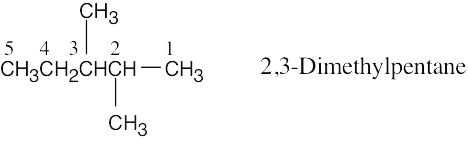
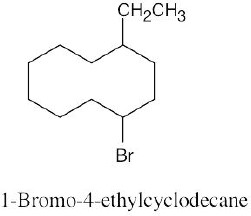
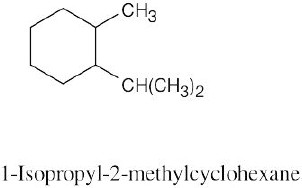
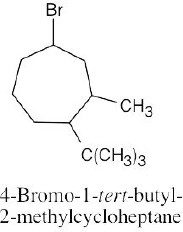
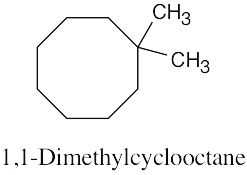
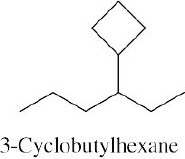
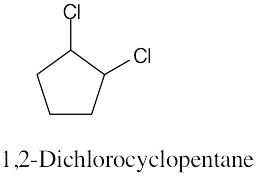
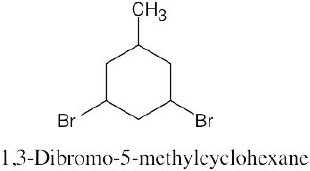
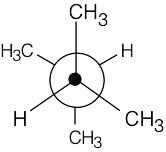
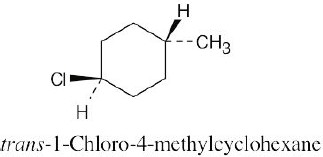
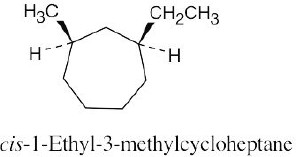
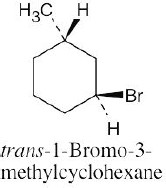
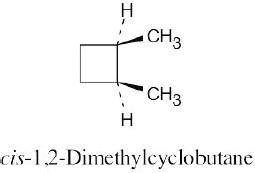
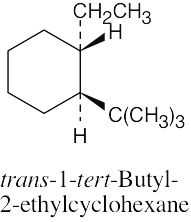
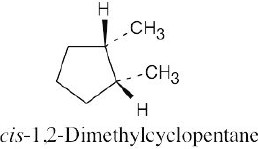
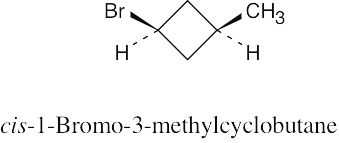
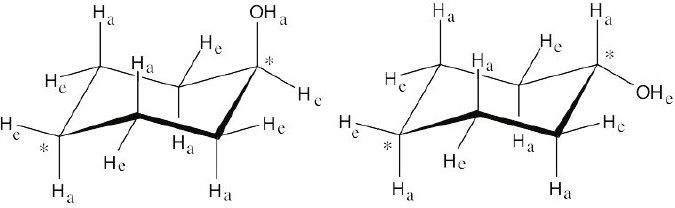
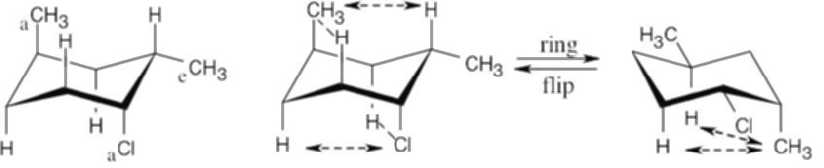 Left conformation: 2 [H ↔ CH3] = 7.6 kJ/mol + 2 [H ↔ Cl]= 2.0 kJ/mol = 9.6 kJ/mol
Left conformation: 2 [H ↔ CH3] = 7.6 kJ/mol + 2 [H ↔ Cl]= 2.0 kJ/mol = 9.6 kJ/mol