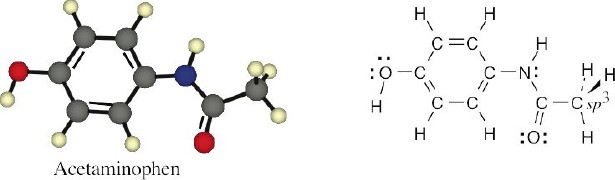
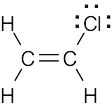
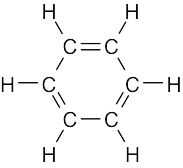
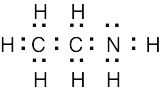
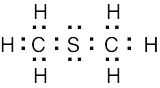
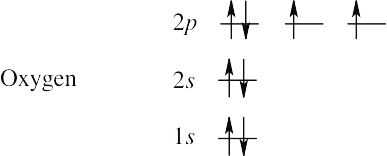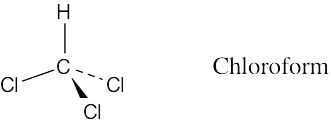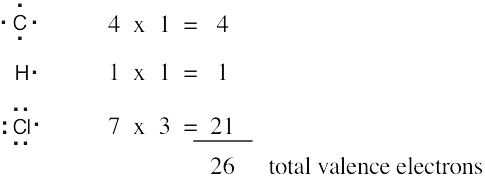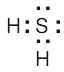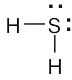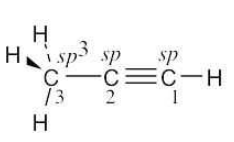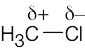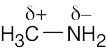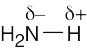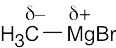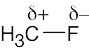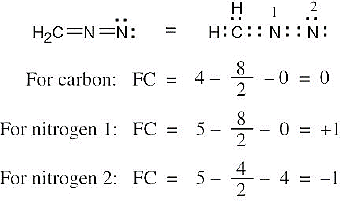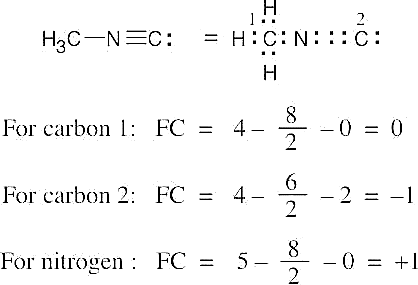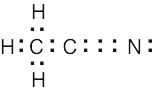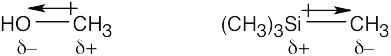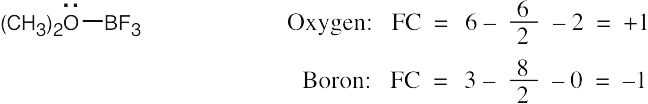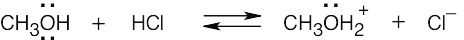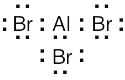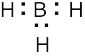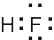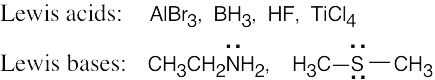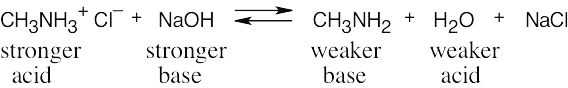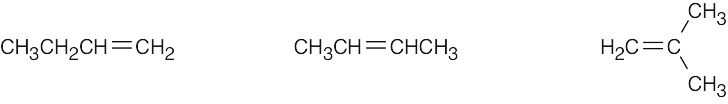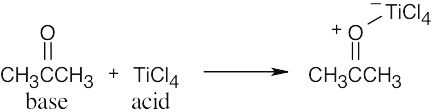1 Chapter 1 Solutions to Problems – Structure and Bonding; Acids and Bases
Chapter 1 – Structure and Bonding; Acids and Bases
Solutions to Problems
| 1.1 | (a) | To find the ground-state electron configuration of an element, first locate its atomic number. For oxygen, the atomic number is 8; oxygen thus has 8 protons and 8 electrons. Next, assign the electrons to the proper energy levels, starting with the lowest level. Fill each level completely before assigning electrons to a higher energy level.
Notice that the 2p electrons are in different orbitals. According to Hund’s rule, we must place one electron into each orbital of the same energy level until all orbitals are half filled. Remember that only two electrons can occupy the same orbital, and that they must be of opposite spin. A different way to represent the ground-state electron configuration is to simply write down the occupied orbitals and to indicate the number of electrons in each orbital. For example, the electron configuration for oxygen is 1s2 2s2 2p4. |
| (b) | Nitrogen, with an atomic number of 7, has 7 electrons. Assign these to energy levels. The more concise way to represent ground-state electron configuration for nitrogen: 1s2 2s2 2p3 | |
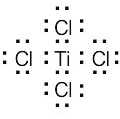
| (c) | Sulfur has 16 electrons. | |
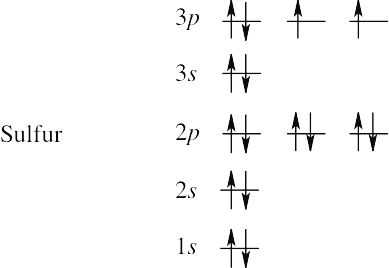 |
||
| 1s2 2s2 2p6 3s6 3p4 |
| 1.2 | The elements of the periodic table are organized into groups that are based on the number of outer-shell electrons each element has. For example, an element in group 1A has one outer-shell electron, and an element in group 5A has five outer-shell electrons. To find the number of outer-shell electrons for a given element, use the periodic table to locate its group. | |
| (a) | Magnesium (group 2A) has two electrons in its outermost shell. | |
| (b) | Cobalt is a transition metal, which as two electrons in the 4s subshell, plus seven electrons in its 3d subshell. | |
| (c) | Selenium (group 6A) has six electrons in its outermost shell. | |
| 1.3 | A solid line represents a bond lying in the plane of the page, a wedged bond represents a bond pointing out of the plane of the page toward the viewer, and a dashed bond represents a bond pointing behind the plane of the page.
|
|
| 1.4 |  |
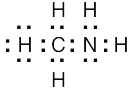
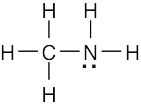
| 1.5 | Identify the group of the central element to predict the number of covalent bonds the element can form | |||||||||||||||||||||
| (a) | Carbon (Group 4A) has four electrons in its valence shell and forms four bonds to achieve the noble-gas configuration of neon. A likely formula is CCl4. | |||||||||||||||||||||
|
||||||||||||||||||||||
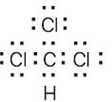
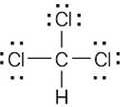
| 1.6 | Start by drawing the electron-dot structure of the molecule. | |||||||||||||||||||||
| (1) |
Determine the number of valence, or outer-shell electrons for each atom in the molecule. For chloroform, we know that carbon has four valence electrons, hydrogen has one valence electron, and each chlorine has seven valence electrons.
|
|||||||||||||||||||||
| (2) |
Next, use two electrons for each single bond.
|
|||||||||||||||||||||
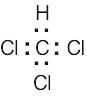
| (3) | Finally, use the remaining electrons to achieve a noble gas configuration for all atoms. For a line-bond structure, replace the electron dots between two atoms with a line | |||||||||||||||||||||
|
||||||||||||||||||||||
| 1.7 | Each of the two carbons has 4 valence electrons. Two electrons are used to form the carbon–carbon bond, and the 6 electrons that remain can form bonds with a maximum of 6 hydrogens. Thus, the formula C2H7 is not possible. |
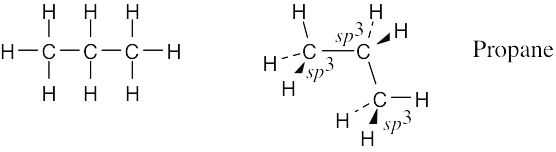
| 1.8 | Connect the carbons and add hydrogens so that all carbons are bonded to four different atoms.
The geometry around all carbon atoms is tetrahedral, and all bond angles are approximately 109°. |
| 1.9 |  |
| 1.10 |
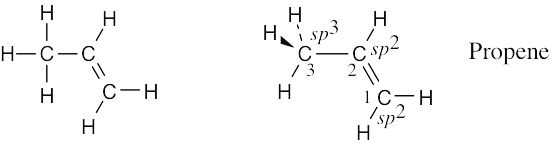
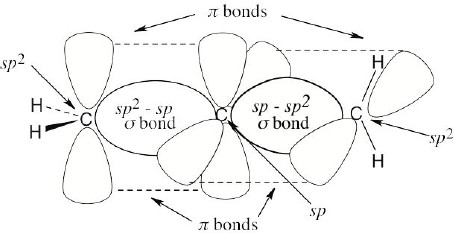
The C2–H and C1–H bonds are σ bonds formed by overlap of an sp2 orbital of carbon with an s orbital of hydrogen. The C2–C3 bond is a σ bond formed by overlap of an sp3 orbital of carbon 3 with an sp2 orbital of carbon 2. There are two C1–C2 bonds. One is a σ bond formed by overlap of an sp3 orbital of carbon 1 with an sp2 orbital of carbon 2. The other is a π bond formed by overlap of a p orbital of carbon 1 with a p orbital of carbon 2. All four atoms connected to the carbon–carbon double bond lie in the same plane, and all bond angles between these atoms are 120°. The bond angle between hydrogen and the sp3-hybridized carbon is 109°. |
| 1.11 | 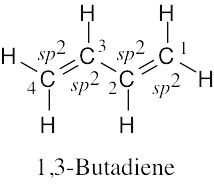 |
All atoms lie in the same plane, and all bond angles are approximately 120°. |
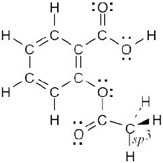
| 1.12 |
|
Aspirin: All carbons are sp2 hybridized, with the exception of the indicated carbon. All oxygen atoms have two lone pairs of electrons. |
| 1.13 |
|
The C3-H bonds are σ bonds formed by overlap of an sp3 orbital of carbon 3 with an s orbital of hydrogen. The C1-H bond is a σ bond formed by overlap of an sp orbital of carbon 1 with an s orbital of hydrogen. The C2-C3 bond is a σ bond formed by overlap of an sp orbital of carbon 2 with an sp3 orbital of carbon 3. There are three C1-C2 bonds. One is a σ bond formed by overlap of an sp orbital of carbon 1 with an sp orbital of carbon 2. The other two bonds are π bonds formed by overlap of two p orbitals of carbon 1 with two p orbitals of carbon 2. The three carbon atoms of propyne lie in a straight line: the bond angle is 180°. The H–C1≡C2 bond angle is also 180°. The bond angle between hydrogen and the sp3-hybridized carbon is 109°. |
| 1.14 | |||
| (a) | 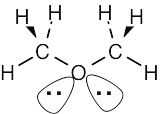 |
The sp3-hybridized oxygen atom has tetrahedral geometry. | |
| (b) | 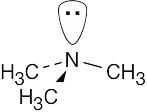 |
Tetrahedral geometry at nitrogen and carbon. | |
| (c) | 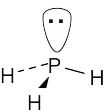 |
Like nitrogen, phosphorus has five outer-shell electrons. PH3 has tetrahedral geometry. | |
| (d) | 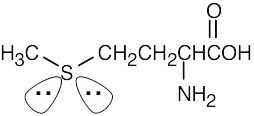 |
The sp3-hybridized sulfur atom has tetrahedral geometry. |
| 1.15 | Remember that the end of a line represents a carbon atom with 3 hydrogens, a two-way intersection represents a carbon atom with 2 hydrogens, a three-way intersection represents a carbon with 1 hydrogen and a four-way intersection represents a carbon with no hydrogens. | |
| (a) |  |
|
| (b) | 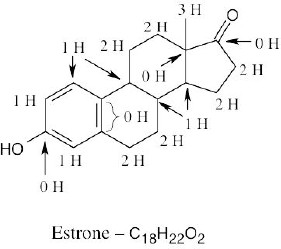 |
|
| 1.16 | Several possible skeletal structures can satisfy each molecular formula. | ||
| (a) | C5H12 | 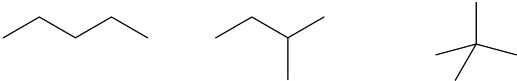 |
|
| (b) | C2H7N |  |
|
| (c) | C3H6O | 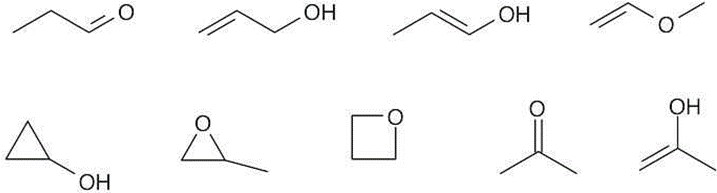 |
|
| (d) | C4H9Cl | 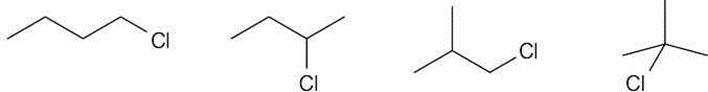 |
|
| 1.17 |  |
| 1.18
After solving this problem, use Figure 1.20 to check your answers. The larger the number, the more electronegative (EN) the element.
|
||||||||||||||||
| Carbon is slightly more electronegative than hydrogen. |
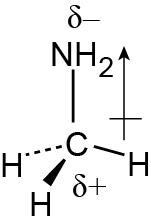
| 1.19 | As in Problem 1.19, use Figure 1.20. The partial negative charge is placed on the more electronegative atom, and the partial positive charge is placed on the less electronegative atom. | ||||||||||||
|
| 1.20 | Use Figure 1.20 to find the electronegativities of each element. Calculate ΔEN and rank the answers in order of increasing ΔEN. | ||||||||||||||||||||||||||||||||||||||||||
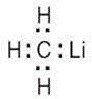
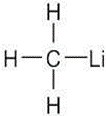
The most polar bond has the largest ΔEN. Thus, in order of increasing bond polarity: H3C — OH < H3C — MgBr < H3C — Li, H3C — F < H3C — K |
| 1.21 | In an electrostatic potential map, the color red indicates regions of a molecule that are electron-rich. The map shows that nitrogen is the most electronegative atom in chloromethane, and the direction of polarity of the C–N bond is:
|
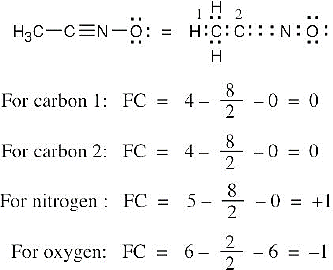
| 1.22 | To find the formal charge of an atom in a molecule, follow these two steps:
(1) Draw an electron-dot structure of the molecule. (2) Use the formula in Section 1.14 (shown below) to determine formal charge for each atom. The periodic table shows the number of valence electrons of the element, and the electron-dot structure shows the number of bonding and nonbonding electrons.
|
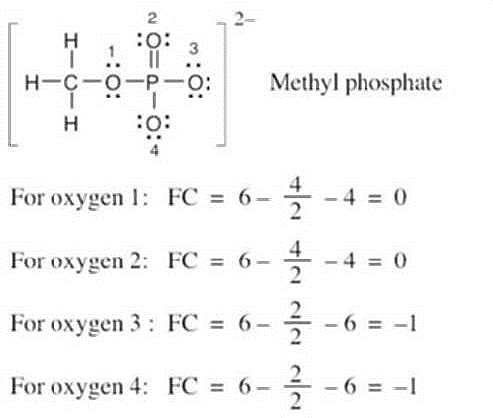
| 1.23 | 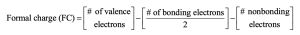
Oxygen atoms 3 and 4 each have a formal charge of –1, and oxygen atoms 1 and 2 have a formal charge of 0. |
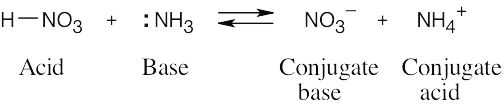
| 1.24 | When an acid loses a proton, the product is the conjugate base of the acid. When a base gains a proton, the product is the conjugate acid of the base.
|
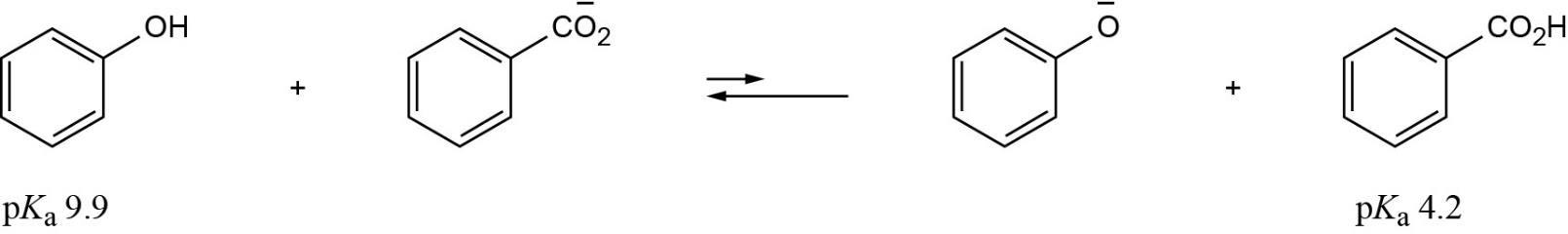
| 1.25 | Recall from Section 1.18 that a stronger acid has a smaller pKa and a weaker acid has a larger pKa. Accordingly, phenylalanine (pKa = 1.83) is a stronger acid than tryptophan (pKa = 2.83). |
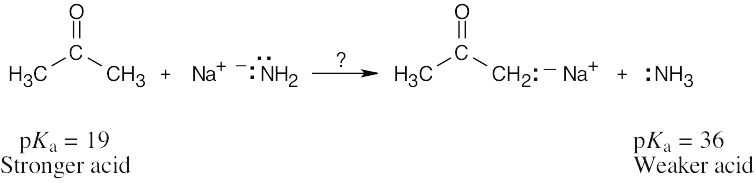
| 1.26 | HO–H is a stronger acid than H2N–H. Since H2N– is a stronger base than HO–, the conjugate acid of H2N– (H2N–H) is a weaker acid than the conjugate acid of HO– (HO–H). |
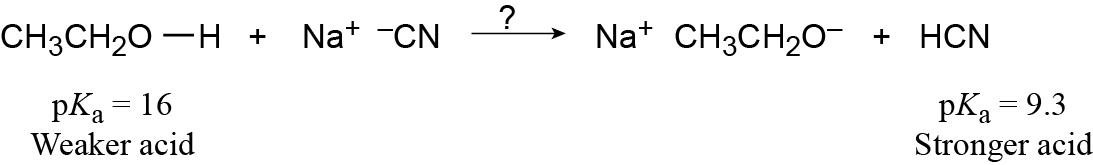
| 1.27 | Use Table 1.6 to find the strength of each acid. A reaction takes place as written if the stronger acid is the reactant.
|
| 1.28 |
As written, the above reaction will take place to virtual completion due to the large difference in pKa values. |
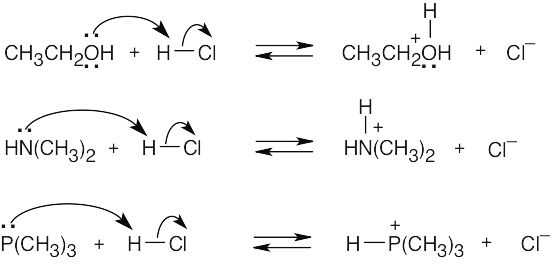
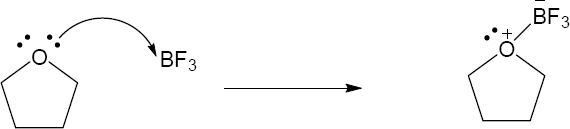
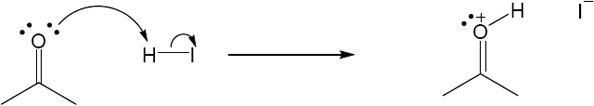
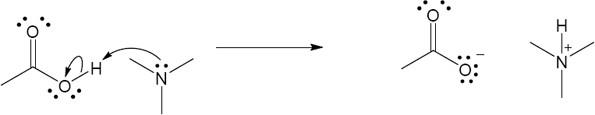
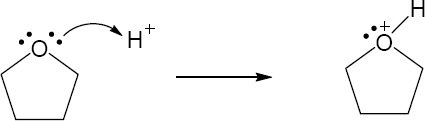
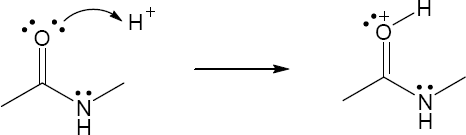
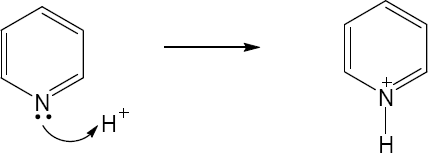
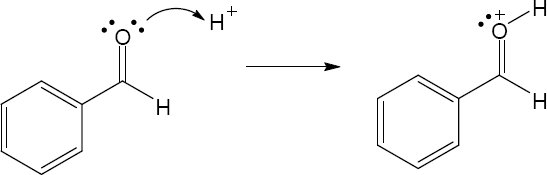
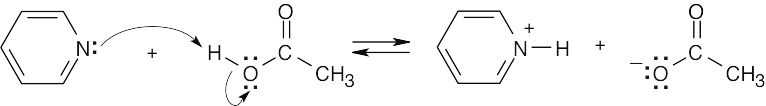
| 1.29 | Locate the electron pair(s) of the Lewis base and draw a curved arrow from the electron pair to the Lewis acid. The electron pair moves from the atom at the tail of the arrow (Lewis base) to the atom at the point of the arrow (Lewis acid). (Note: electron dots have been omitted from Cl– to reduce clutter.)
|
Additional Problems
Visualizing Chemistry
| 1.30 | (a) | 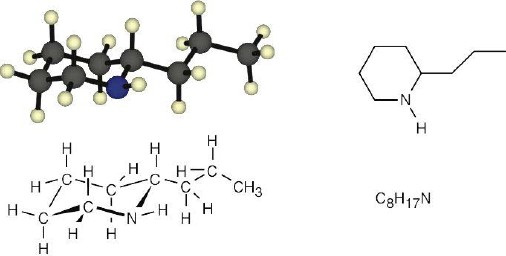 |
| (b) | 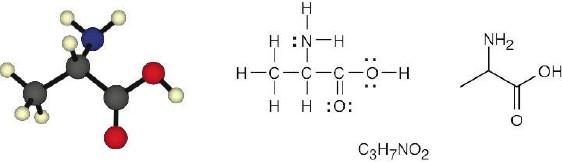 |
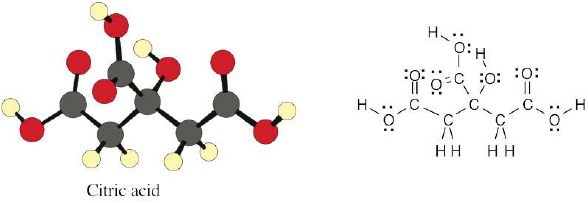
| 1.31 | Citric acid (C6H8O7) contains seven oxygen atoms, each of which has two electron lone pairs. Three of the oxygens form double bonds with carbon.
|
| 1.32 | All carbons are sp2 hybridized, except for the carbon indicated as sp3. The two oxygen atoms and the nitrogen atom have lone pair electrons, as shown.
|
| 1.33 | 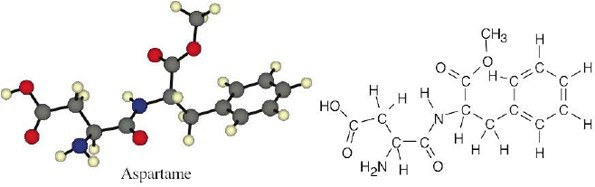 |
Electron Configuration
| 1.34 | Element | Number | Number of valence electrons | ||
| (a) | Zinc | 30 | 2 | ||
| (b) | Iodine | 53 | 7 | ||
| (c) | Silicon | 14 | 4 | ||
| (d) | Iron | 26 | 2 (in 4s subshell), 6 (in 3d subshell) |
| 1.35 | Element | Number | Ground-state electron configuration | ||
| (a) | Potassium | 19 | 1s2 2s2 2p6 3s2 3p6 4s1 | ||
| (b) | Arsenic | 33 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 | ||
| (c) | Aluminum | 13 | 1s2 2s2 2p6 3s2 3p1 | ||
| (d) | Germanium | 32 | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 |
Electron-Dot and Line-Bond Structures
| 1.36 | (a) | NH2O | (b) | AlCl3 | (c) | CF2Cl2 | (d) | CH2O |
| 1.37 | (a) | The 4 valence electrons of carbon can form bonds with a maximum of 4 hydrogens. Thus, it is not possible for the compound CH5 to exist. |
| (b) | If you try to draw a molecule with the formula C2H6N, you will see that it is impossible for both carbons and nitrogen to have a complete octet of electrons. Therefore, C2H6N is unlikely to exist. | |
| (c) | A compound with the formula C3H5Br2 doesn’t have filled outer shells for all atoms and is thus unlikely to exist. |
| 1.38 | In the compound acetonitrile, nitrogen has eight electrons in its outer electron shell. Six are used in the carbon-nitrogen triple bond, and two are a nonbonding electron pair.
Acetonitrile |
| 1.39 | Vinyl chloride has 18 valence electrons. Eight electrons are used for 4 single bonds, 4 electrons are used in the carbon–carbon double bond, and 6 electrons are in the 3 lone pairs that surround chlorine.
Vinyl chloride |
| 1.40 | (a) | (b) | (c) | |||
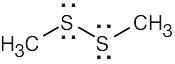 |
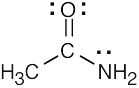 |
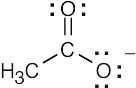 |
| 1.41 | In molecular formulas of organic molecules, carbon is listed first, followed by hydrogen. All other elements are listed in alphabetical order. | |||
| Compound | Molecular formula | |||
| (a) | Aspirin | C9H8O4 | ||
| (b) | Vitamin C | C6H8O6 | ||
| (c) | Nicotine | C10H14N2 | ||
| (d) | Glucose | C6H12O6 | ||
| 1.42 | To work a problem of this sort, you must draw all possible structures consistent with the rules of valence. You must systematically consider all possible attachments, including those that have branches, rings and multiple bonds. |
|
| (a) | 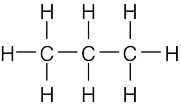 |
|
| (b) | 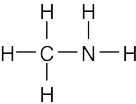 |
|
| (c) | 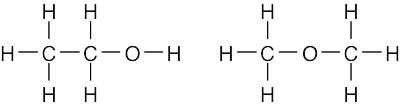 |
|
| (d) | 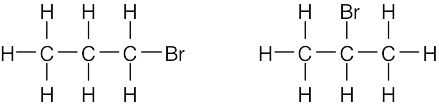 |
|
| (e) | 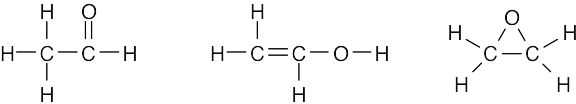 |
|
| (f) | 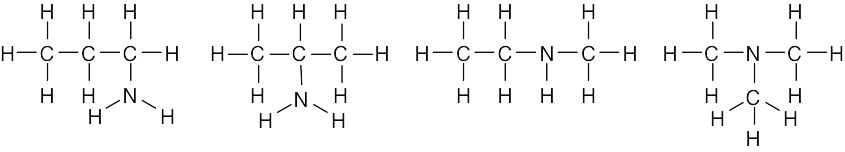 |
|
| 1.43 | 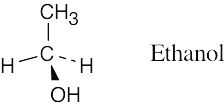 |
| 1.44 | 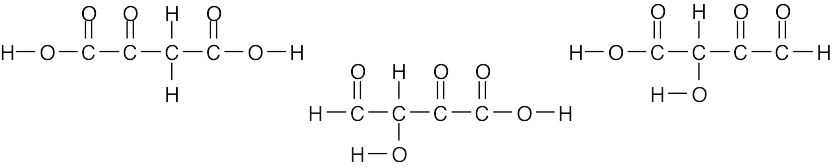 |
| 1.45 | (a) | 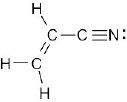 |
| (b) | 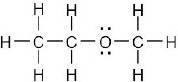 |
|
| (c) | 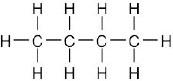 |
|
| (d) | 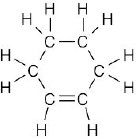 |
Hybridization
| 1.46 | The H3C– carbon is sp3 hybridized, and the –CN carbon is sp hybridized. |
| 1.47 | (a) | 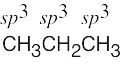 |
| (b) | 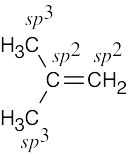 |
|
| (c) | 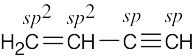 |
|
| (d) | 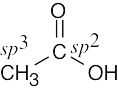 |
| 1.48 | All carbon atoms of benzene are sp2 hybridized, and all bond angles of benzene are 120°. Benzene is a planar molecule.
Benzene |
| 1.49 | (a) | 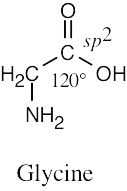 |
| (b) | 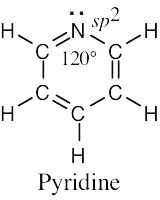 |
|
| (c) | 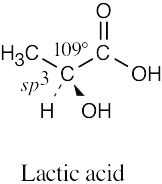 |
| 1.50 | Examples: | |
| (a) | CH3CH2CH=CH2 | |
| (b) | H2C=CH–CH=CH2 | |
| (c) | H2C=CH–C≡CH | |
| 1.51 | (a) | 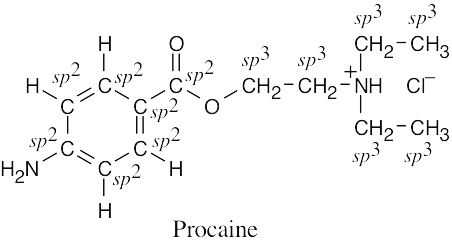 |
| (b) |  |
| 1.52 | The bond angles formed by atoms having sp3 hybridization are approximately 109°. The bond angles formed by atoms having sp2 hybridization are approximately 120°.
|
Skeletal Structures
| 1.53 | (a) |  |
| (b) |  |
|
| (c) |  |
|
| (d) |  |
| 1.54 | (a) | 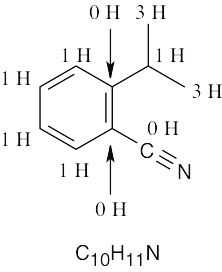 |
| (b) | 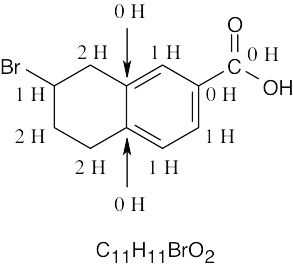 |
|
| (c) | 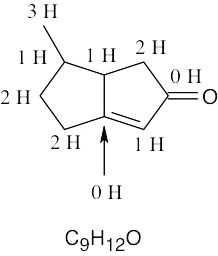 |
| 1.55 | 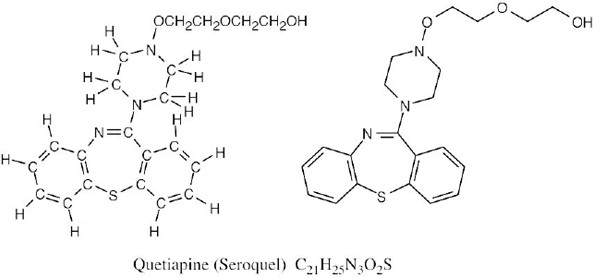 |
| 1.56 | 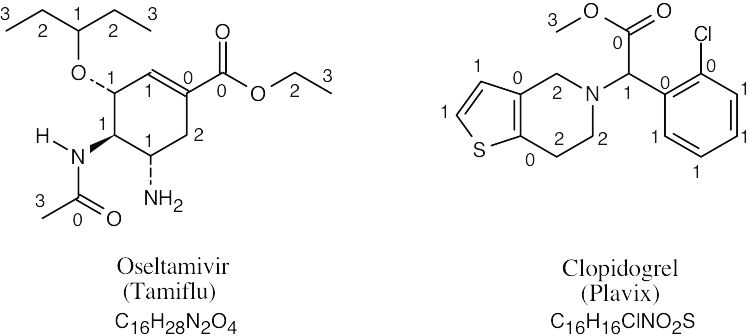 |
Mechanism Problems
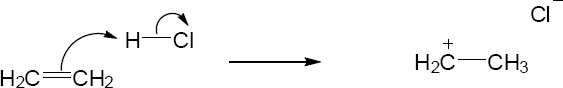
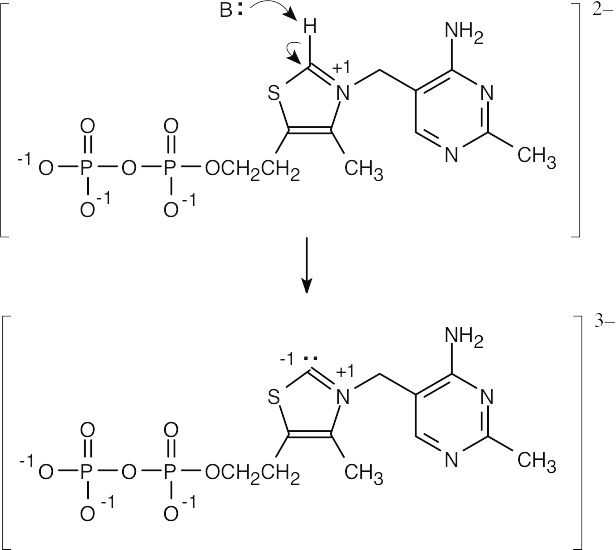
| 1.57 |
|
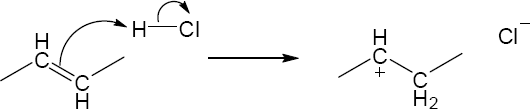
| 1.58 |
|
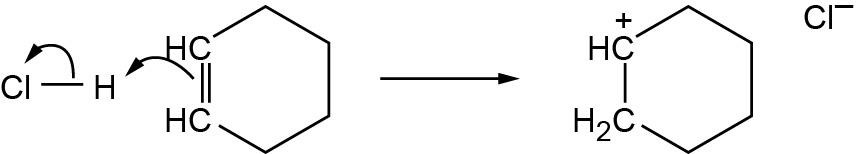
| 1.59 |
|
Electronegativity and Dipole Moments
| 1.60 | Use Figure 2.3 if you need help. The most electronegative element is underlined.
|
| 1.61 |
|
Formal Charges
| 1.62 | To save space, molecules are shown as line-bond structures with lone pairs, rather than as electron-dot structures.
|
| 1.63 | Molecules are shown as line-bond structures with lone-pair electrons indicated. Only calculations for atoms with non-zero formal charge are shown.
|
Resonance
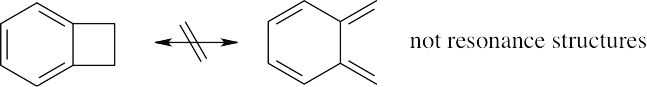
| 1.64 | Resonance forms do not differ in the position of nuclei. The two structures in (a) are not resonance forms because the positions of the carbon and hydrogen atoms outside the ring are different in the two forms.
The pairs of structures in parts (b), (c), and (d) represent resonance forms. |
Acids and Bases
| 1.65 |
|
| 1.66
|
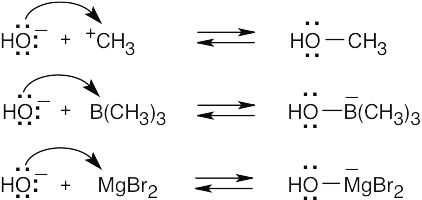
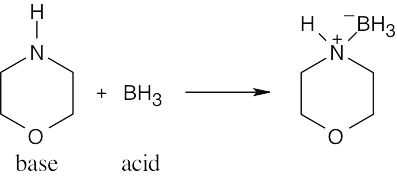
The Lewis acids shown below can accept an electron pair either because they have a vacant orbital or because they can donate H+. The Lewis bases have nonbonding electron pairs.
|
| 1.67 |
|
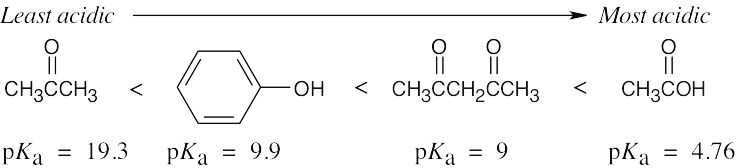
| 1.68 | The substances with the largest values of pKa are the least acidic.
|
| 1.69 | To react completely (> 99.9%) with NaOH, an acid must have a pKa at least 3 units smaller than the pKa of H2O. Thus, all substances in the previous problem except acetone react completely with NaOH. |
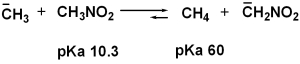
| 1.70 | The stronger the acid (smaller pKa), the weaker its conjugate base. Since NH4+ is a stronger acid than CH3NH3+, CH3NH2 is a stronger base than NH3. |
| 1.71 |
|
| 1.72 | Only acetic acid will react with sodium bicarbonate. Acetic acid is the only substance in Problem 1.68 that is a stronger acid than carbonic acid. |
General Problems
| 1.73 | In a compound containing a carbon–carbon triple bond, atoms bonded to the sp-hybridized carbons must lie in a straight line. It is not possible to form a five-membered ring if four carbons must have a linear relationship. |
| 1.74 | The central carbon of allene forms two σ bonds and two π bonds. The central carbon is sp-hybridized, and the two terminal carbons are sp2-hybridized. The bond angle formed by the three carbons is 180°, indicating linear geometry for the carbons of allene.
|
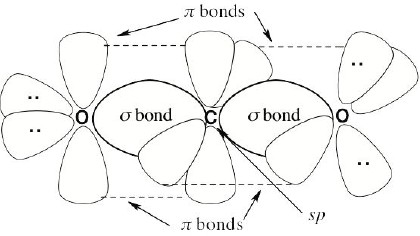
| 1.75 | Carbon dioxide is a linear molecule.
|
| 1.76 | All of the indicated atoms are sp2-hybridized.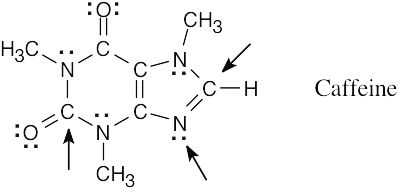 |
| 1.77 | (a) | The positively charged carbon atom is surrounded by six valence electrons; carbon has three valence electrons, and each hydrogen brings three valence electrons. |
| (b) | The positively charged carbon is sp2-hybridized. | |
| (c) | A carbocation is planar about the positively charged carbon. |
| 1.78 | 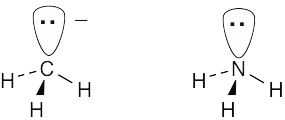 |
|
| (a) | A carbanion is isoelectronic with (has the same number of electrons as) a trivalent nitrogen compound. | |
| (b) | The negatively charged carbanion carbon has eight valence electrons. | |
| (c) | The carbon atom is sp3-hybridized. | |
| (d) | A carbanion is tetrahedral. | |
| 1.79 | The two compounds differ in the way that the carbon atoms are connected.
|
| 1.80 | One compound has a double bond, and one has a ring.
|
| 1.81 | The two compounds differ in the location of the oxygen atom.
CH3CH2OH CH3OCH3 |
| 1.82 | The compounds differ in the way that the carbon atoms are connected and in the location of the double bond.
|
| 1.83 | (a) | 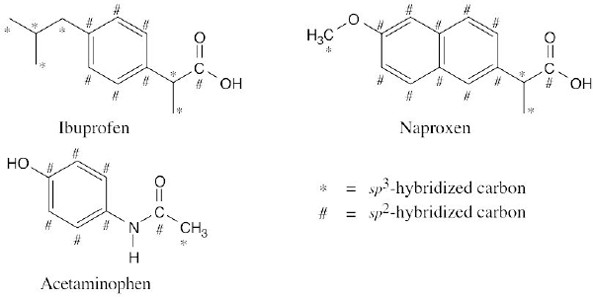 |
||||||||||||
| (b) |
|
|||||||||||||
| (c) | Each of the structures has a six-membered ring containing three double bonds, each has a methyl group, and each has a C=O group. |
| 1.84 | Reactions (a) and (c) are reactions between Brønsted–Lowry acids and bases; the stronger acid and stronger base are identified. Reactions (b) and (d) occur between Lewis acids and bases.
|
| 1.85 | Pairs (a) and (d) represent resonance structures; pairs (b) and (c) do not. For two structures to be resonance forms, all atoms must be in the same positions in all resonance forms. |
| 1.86 |
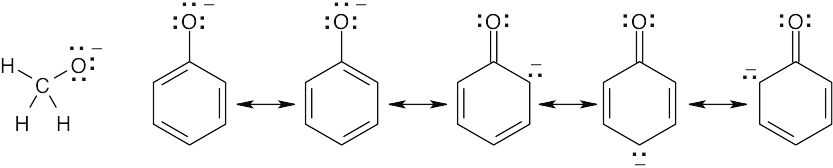
When phenol loses a proton, the resulting anion is stabilized by resonance. The methanol anion is not stabilized by resonance. |
| 1.87 |
|
| 1.88 | The equilibrium always favors the weaker acid/base pair (acid with the higher pKa).
|