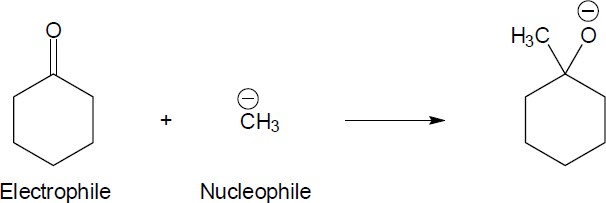
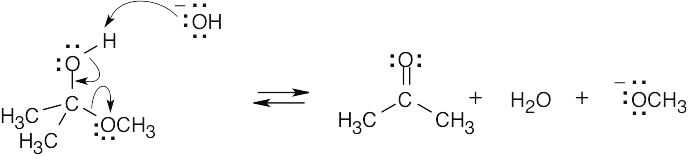
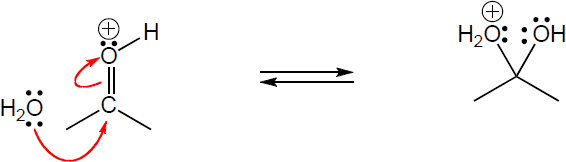
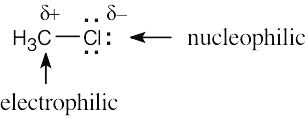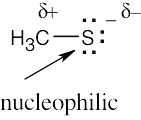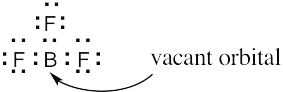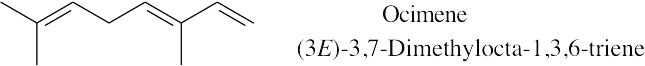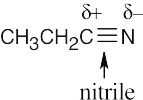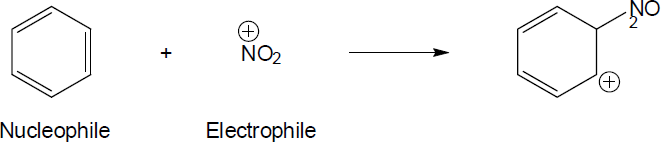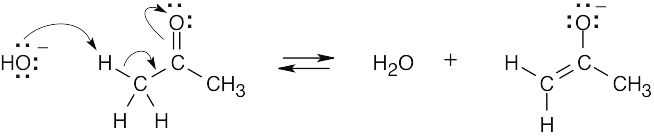4 Chapter 4 Solutions to Problems – An Overview of Organic Reactions
Chapter 4 – An Overview of Organic Reactions
Solutions to Problems
| 4.1 | (1) | Find the longest chain containing the double bond and name it. In (a), the longest chain is a pentene. |
| (2) | Identify the substituents. There are three methyl groups in (a). | |
| (3) | Number the substituents, remembering that the double bond receives the lowest possible number. The methyl groups are attached to C3 and C4 (two methyl groups). | |
| (4) | Name the compound, remembering to use the prefix “tri-” before “methyl” and remembering to use a number to signify the location of the double bond. The name of the compound in (a) is 3,4,4-trimethyl-1-pentene. | |
| (a) | 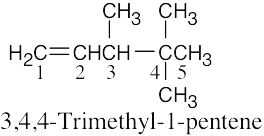 |
|
| (b) | 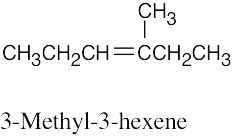 |
|
| (c) | 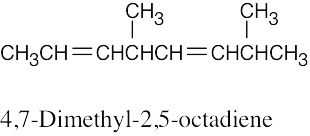 |
|
| (d) | 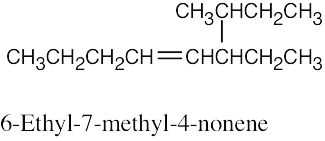 |
| 4.2 | It is much easier to draw a structure from a given name than it is to name a structure. First, draw the carbon chain, placing the double bond or bonds in the designated locations. Then attach the cited groups in the proper positions. | |
| (a) | 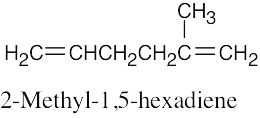 |
|
| (b) | 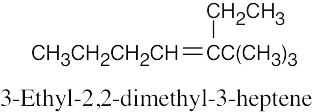 |
|
| (c) | 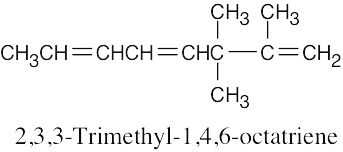 |
|
| (d) | 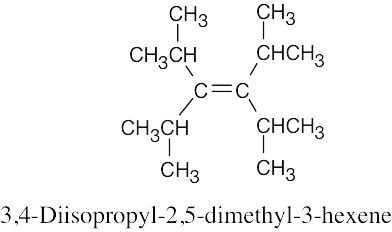 |
|
| 4.3 | (a) | 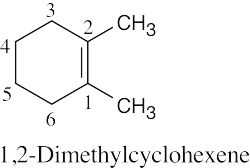 |
| (b) | 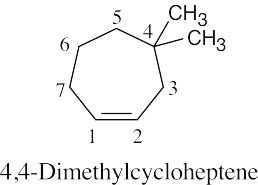 |
|
| (c) | 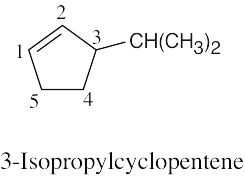 |
| 4.4 | In the new naming system, the bond locant appears directly before –ene or –diene. | |
| (a) | 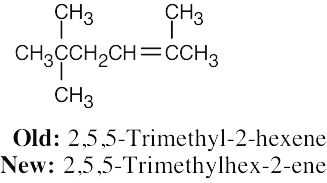 |
|
| (b) | 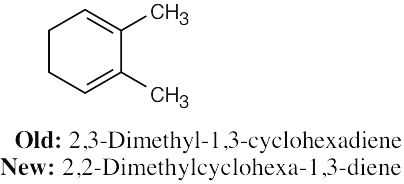 |
|
| 4.5 | The rules for naming alkynes are almost the same as the rules for naming alkenes. The suffix –yne is used, and compounds containing both double bonds and triple bonds are –enynes, with the double bond taking numerical precedence. | |
| (a) | 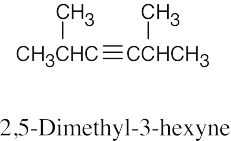 |
|
| (b) | 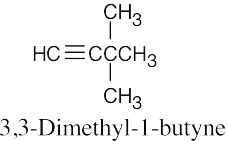 |
|
| (c) | 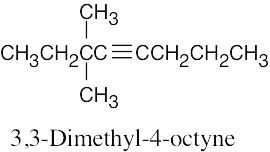 |
|
| (d) | 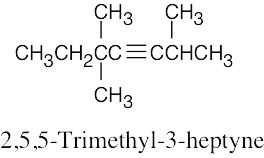 |
|
| (e) | 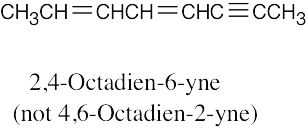 |
|
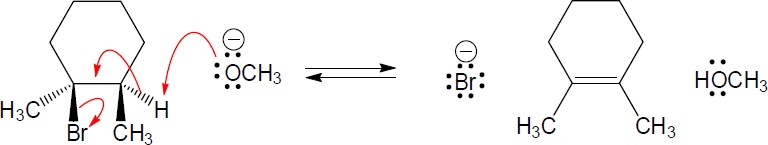
| 4.6 | 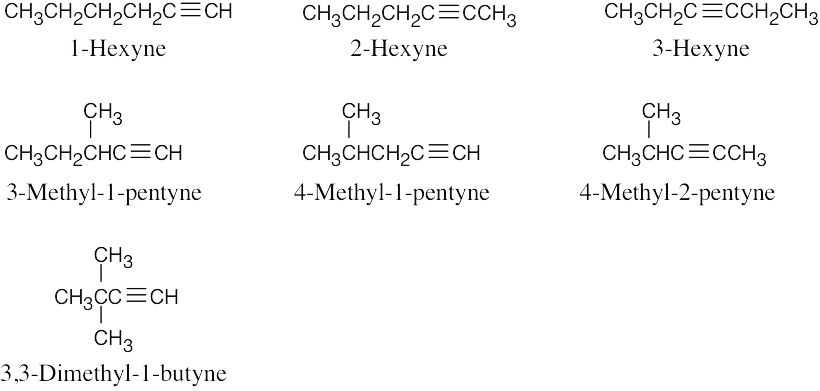 |
| 4.7 | Compounds (c), (e), and (f) can exist as cis–trans isomers. | |
| (c) | 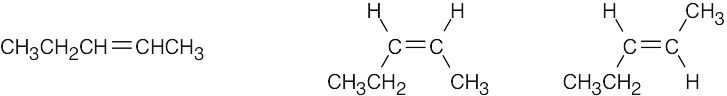 |
|
| (e) | 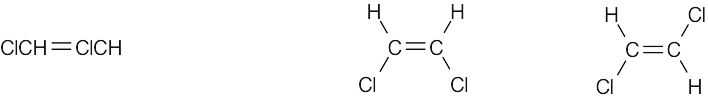 |
|
| (f) | 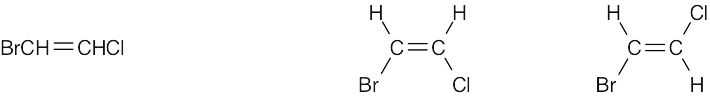 |
|
| 4.8 | (a) | 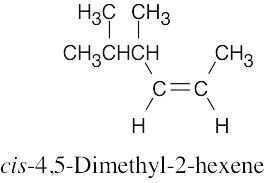 |
| (b) | 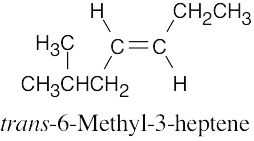 |
| 4.9 | Review the sequence rules presented in Section 4.4. A summary: | |||||||||||||||||||||||||||||
| Rule 1: | An atom with a higher atomic number has priority over an atom with a lower atomic number. | |||||||||||||||||||||||||||||
| Rule 2: | If a decision can’t be reached by using Rule 1, look at the second, third, or fourth atom away from the double-bond carbon until a decision can be made. | |||||||||||||||||||||||||||||
| Rule 3: | Multiple-bonded atoms are equivalent to the same number of single-bonded atoms. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| 4.10 | Highest priority ⎯⎯⎯→ Lowest Priority | |
| (a) | –Cl, –OH, –CH3, –H | |
| (b) | –CH2OH, –CH=CH2, –CH2CH3, –CH3 | |
| (c) | –CO2H, –CH2OH, –C≡N, –CH2NH2 | |
| (d) | –CH2OCH3, –C≡N, –C≡CH, –CH2CH3 | |
| 4.11 | (a) | 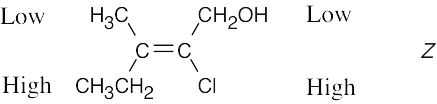
First, consider the substituents on the right side of the double bond. –Cl ranks higher than –CH2OH by Rule 1 of the Cahn–Ingold–Prelog rules. On the left side of the double bond, –CH2CH3 ranks higher than –CH3. The isomer is Z when the two higher priority groups lie on the same side of the double bond. Otherwise, the isomer is E. |
| (b) | 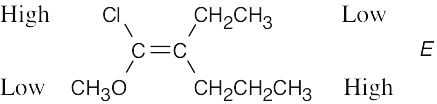 |
|
| (c) | 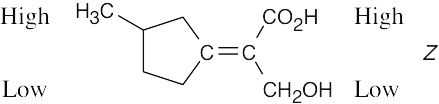
Notice that the upper substituent on the left side of the double bond is of higher priority because of the methyl group attached to the ring. |
|
| (d) | 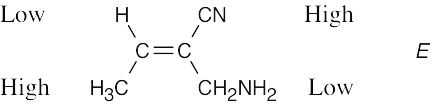 |
| 4.12 | 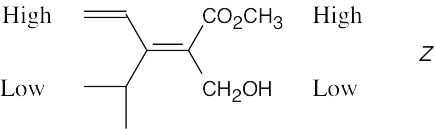 |
| 4.13 |
|
| 4.14 | Keep in mind:
|
| 4.15 | BF3 is likely to be an electrophile because the electrostatic potential map indicates that it is electron-poor (blue). The electron-dot structure shows that BF3 lacks a complete electron octet and can accept an electron pair from a nucleophile.
|
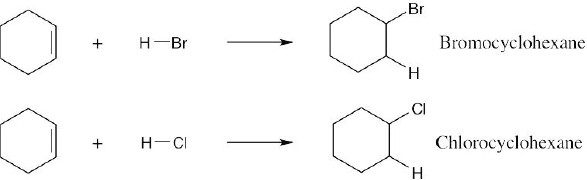
| 4.16 | Reaction of cyclohexene with HCl or HBr is an electrophilic addition reaction in which halogen acid adds to a double bond to produce a haloalkane.
|
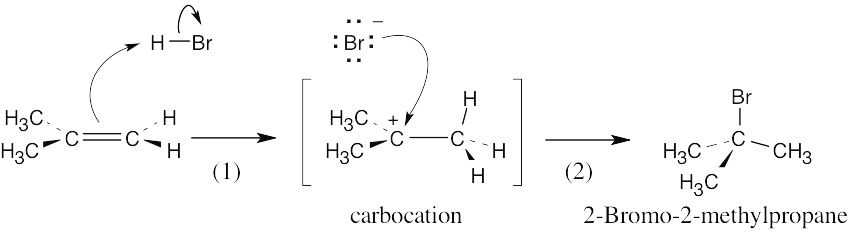
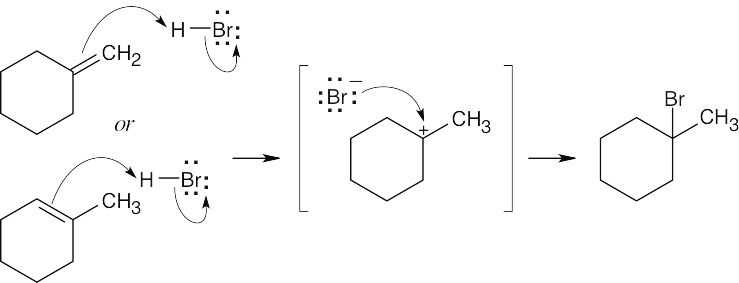
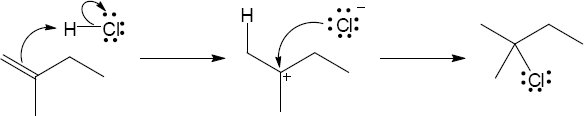
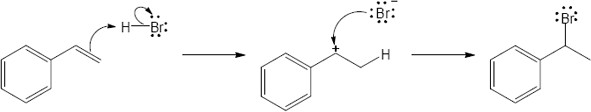
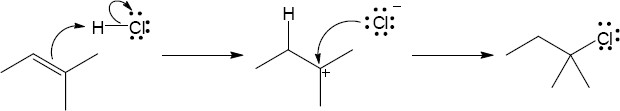
| 4.17 | The mechanism is pictured in Figure 4.7.
The steps: (1) Attack of the π electrons of the double bond on HBr, forming a carbocation (2) Formation of a C–Br bond by electron pair donation from Br– to form the neutral addition product
|
| 4.18 | A reaction with ΔG‡ = 45 kJ/mol is faster than a reaction with ΔG‡ = 70 kJ/mol because a larger value for ΔG‡ indicates a slower reaction. |
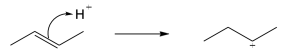
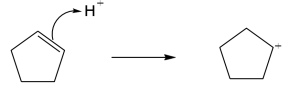
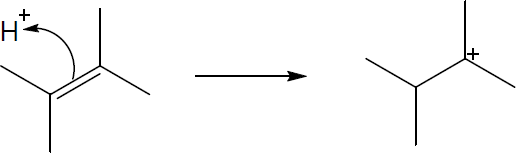
| 4.19 |
|
| 4.20 | For curved arrow problems, follow these steps:
(1) Locate the bonding changes. In (a), a bond from nitrogen to chlorine has formed, and a Cl-Cl bond has broken. (2) Identify the nucleophile and electrophile (in (a), the nucleophile is ammonia and the electrophile is one Cl in the Cl2 molecule), and draw a curved arrow whose tail is near the nucleophile and whose head is near the electrophile. (3) Check to see that all bonding changes are accounted for. In (a), we must draw a second arrow to show the unsymmetrical bond-breaking of Cl2 to form Cl–.
|
| 4.21 | This mechanism will be studied in a later chapter.
|
Additional Problems
Visualizing Chemistry
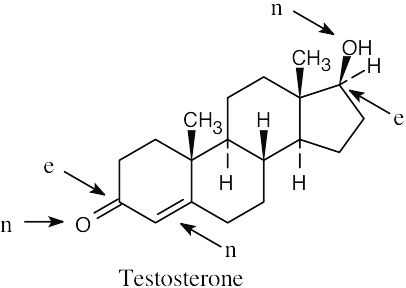
| 4.22 | (a) | 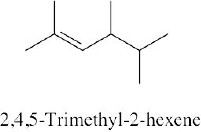 |
| (b) | 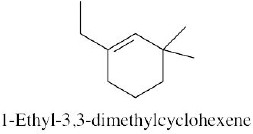 |
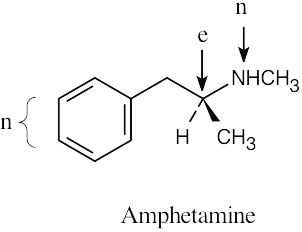
| 4.23 | (a) | 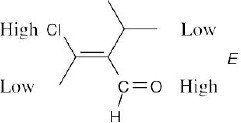 |
| (b) | 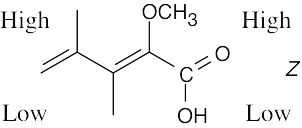 |
| 4.24 |
|
| 4.25 |
|
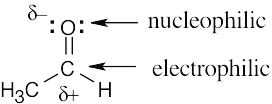
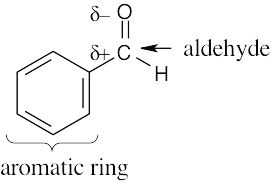
| 4.26 | (a) | The electrostatic potential map shows that the formaldehyde oxygen is electron-rich, and the carbon–oxygen bond is polarized. The carbon atom is thus relatively electron- poor and is likely to be electrophilic. |
| (b) | The sulfur atom is more electron-rich than the other atoms of methanethiol and is likely to be nucleophilic. |
| 4.27 |
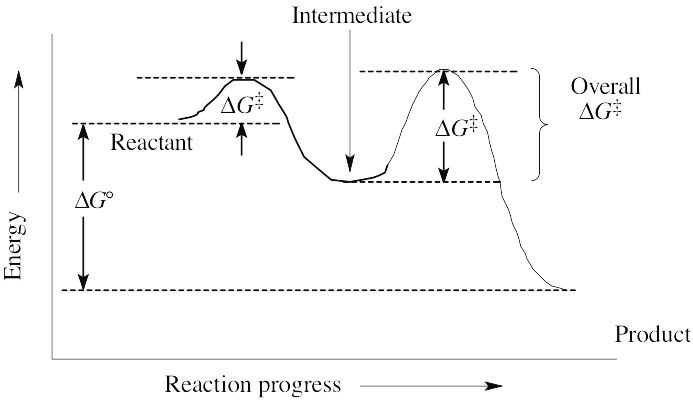
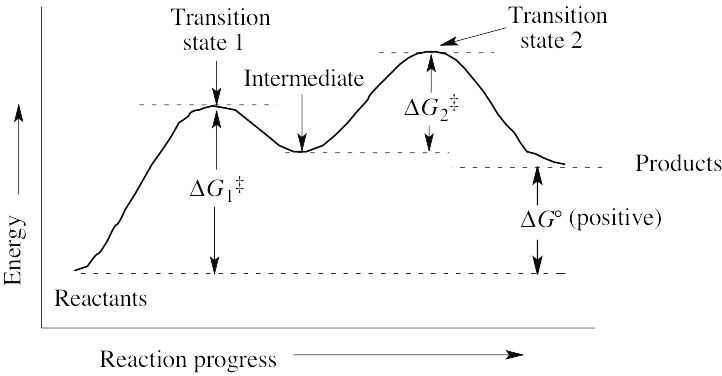
(a) ΔG° is positive. (b) There are two steps in the reaction. (c) There are two transition states, as indicated on the diagram. |
| 4.28 |
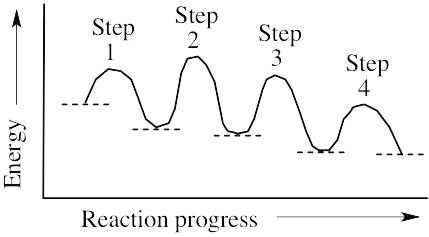
(a) The reaction involves four steps, noted above. (b) Step 1 is the most exergonic because the energy difference between reactant and product (ΔG°) is greatest. (c) Step 2 is slowest because it has the largest value of ΔG‡. |
Naming Alkenes
| 4.29 | (a) | 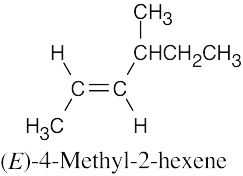 |
| (b) | 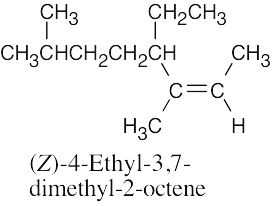 |
|
| (c) | 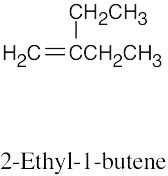 |
|
| (d) | 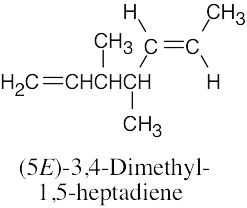 |
|
| (e) | 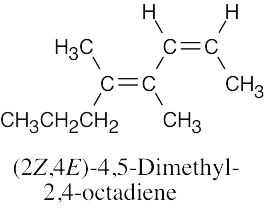 |
|
| (f) | 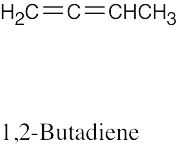 |
| 4.30 | (a) | 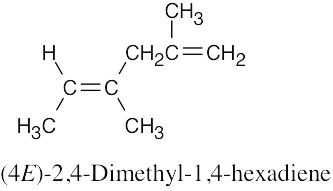 |
| (b) | 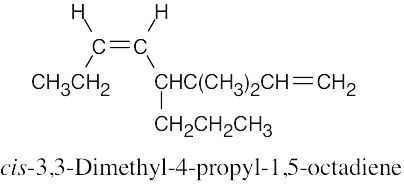 |
|
| (c) | 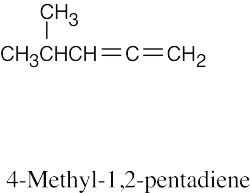 |
|
| (d) | 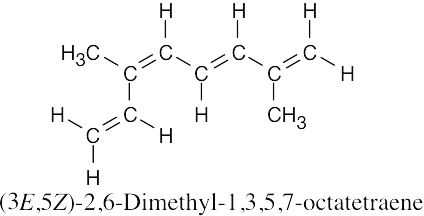 |
|
| (e) | 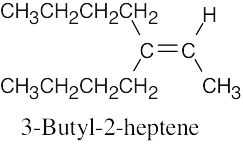 |
|
| (f) | 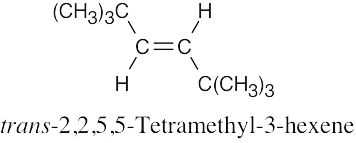 |
| 4.31 | (a) | 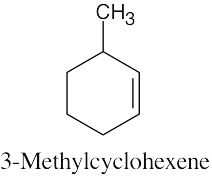 |
| (b) | 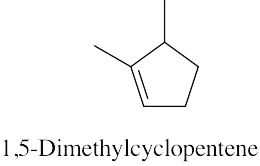 |
|
| (c) | 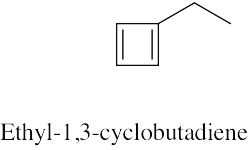 |
|
| (d) | 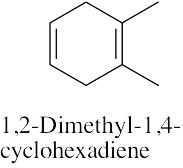 |
|
| (e) | 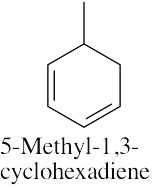 |
|
| (f) | 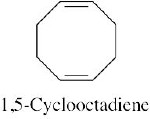 |
| 4.32 |
Because the longest carbon chain contains 8 carbons and 3 double bonds, ocimene is an octatriene. Start numbering at the end that will give the lower number to the first double bond (1,3,6 is lower than 2,5,7). Number the methyl substituents and, finally, name the compound.
|
| 4.33 | 
(3E,6E)-3,7,11-Trimethyl-1,3,6,10-dodecatetraene |
| 4.34 | 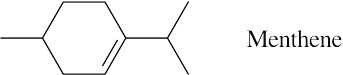 |
| 4.35 | 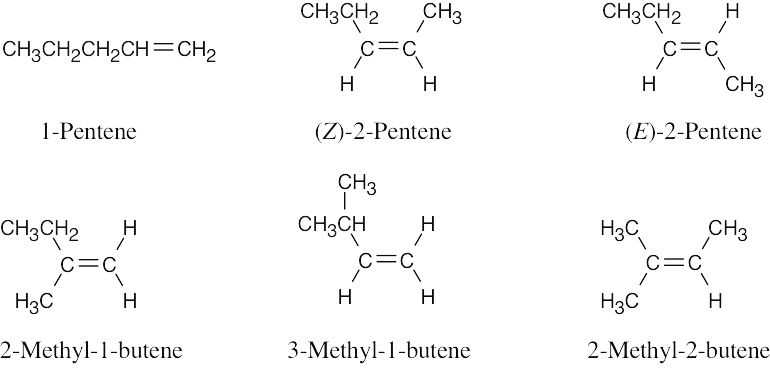 |
Naming Alkynes
| 4.36 | (a) | 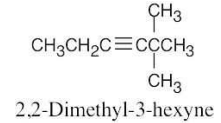 |
| (b) | 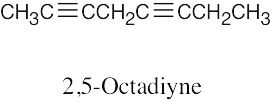 |
|
| (c) | 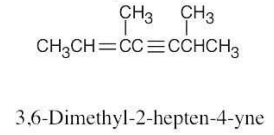 |
|
| (d) | 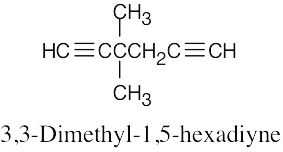 |
|
| (e) | 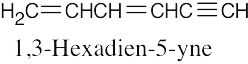 |
|
| (f) | 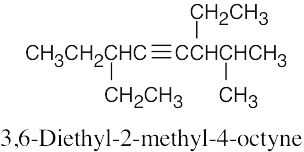 |
| 4.37 | (a) | 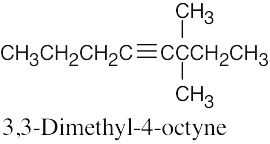 |
| (b) | 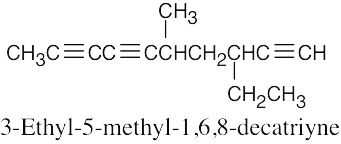 |
|
| (c) | 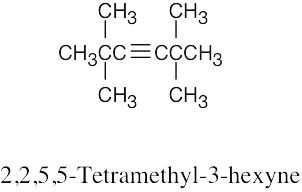 |
|
| (d) | 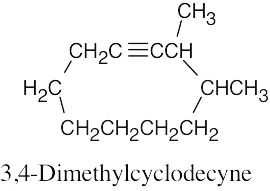 |
|
| (e) | 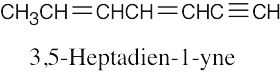 |
|
| (f) | 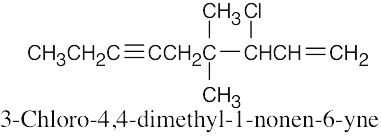 |
|
| (g) | 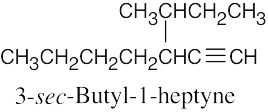 |
|
| (h) | 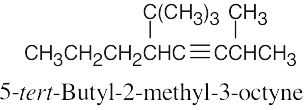 |
| 4.38 | (a) | CH3CH=CHC≡CC≡CCH=CHCH=CHCH=CH2.
1,3,5,11-Tridecatetraen-7,9-diyne Using E–Z notation: (3E,5E,11E)-1,3,5,11-Tridecatetraen-7,9-diyne The parent alkane of this hydrocarbon is tridecane. |
| (b) | CH3C≡CC≡CC≡CC≡CC≡CCH=CH2. 1-Tridecen-3,5,7,9,11-pentayne This hydrocarbon also belongs to the tridecane family. |
Alkene Isomers and Their Stability
| 4.39 | Highest priority ⎯⎯⎯→ Lowest Priority | |
| (a) | –I, –Br, –CH3, –H | |
| (b) | –OCH3, –OH, –CO2H, –H | |
| (c) | –CO2CH3, –CO2H, –CH2OH, –CH3 | |
| (d) | –COCH3, –CH2CH2OH, –CH2CH3, –CH3 | |
| (e) | –CH2Br, –C≡N, –CH2NH2, –CH=CH2 | |
| (f) | –CH2OCH3, –CH2OH, –CH=CH2, CH2CH3 | |
| 4.40 | (a) | 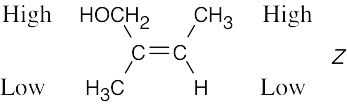 |
| (b) | 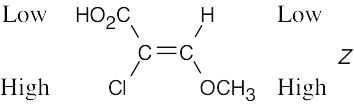 |
|
| (c) | 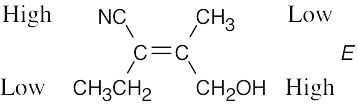 |
|
| (d) | 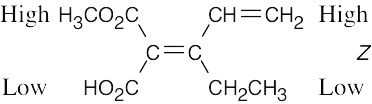 |
| 4.41 | (a) | 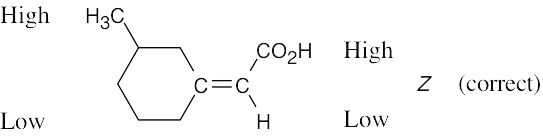 |
| (b) | 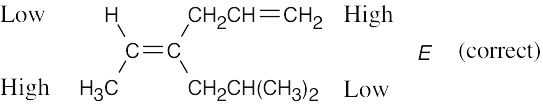 |
|
| (c) | 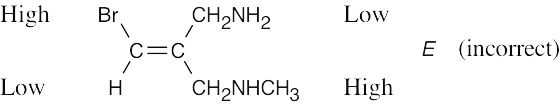 |
|
| (d) | 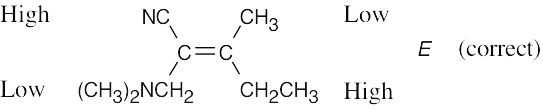 |
|
| (e) | 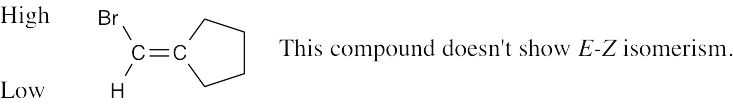 |
|
| (f) | 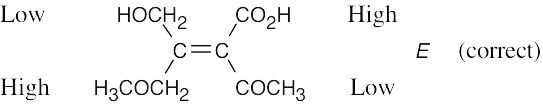 |
| 4.42 |
|
Energy Diagrams and Reaction Mechanisms
| 4.43 | A transition state represents a structure occurring at an energy maximum. An intermediate occurs at an energy minimum between two transition states. Even though an intermediate may be of such high energy that it cannot be isolated, it is still of lower energy than the transition states surrounding it. |
| 4.44 | (a) Mechanism:
(b) Mechanism:
(c) Mechanism:
|
Polar Reactions
| 4.45 |
|
| 4.46 | (a) | The reaction between bromoethane and sodium cyanide is a substitution because two reagents exchange parts. |
| (b) | This reaction is an elimination because two products (cyclohexene and H2O) are produced from one reactant | |
| (c) | Two reactants form one product in this addition reaction. | |
| (d) | This is a substitution reaction. |
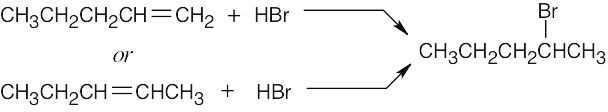
| 4.47 |
|
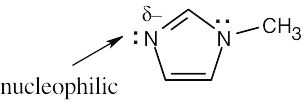
| 4.48 | Nucleophiles are the electron donors in carbon bond forming reactions while the electron acceptors are the electrophiles.
|
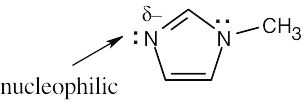
| 4.49 |
|
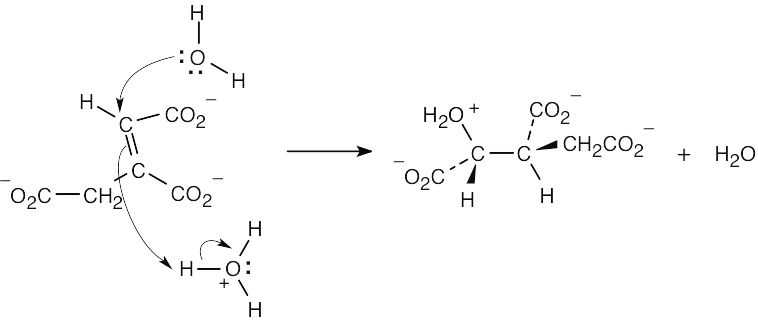
| 4.50 |
|
General Problems
| 4.51 | (a) | 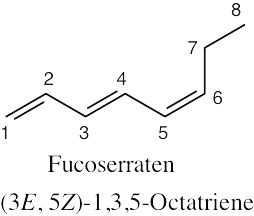 |
| (b) | 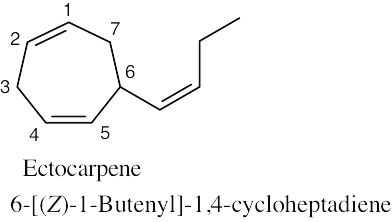 |
| 4.52 | 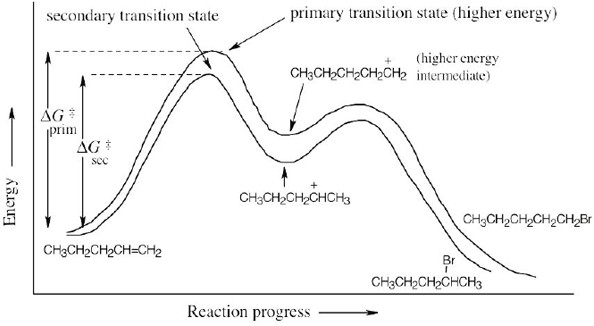 |