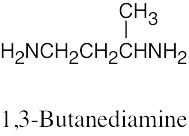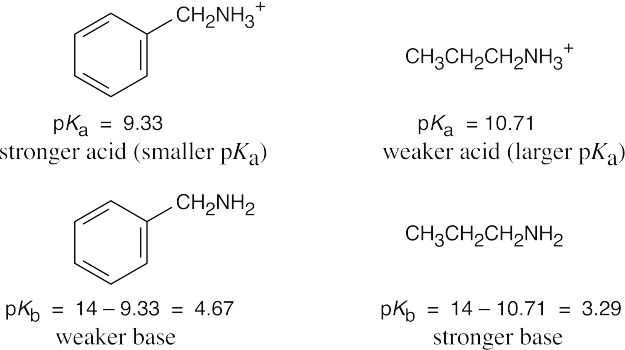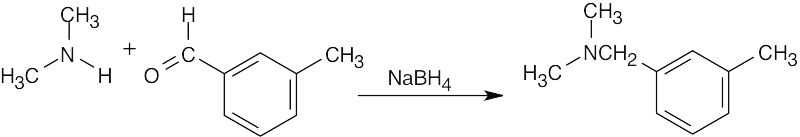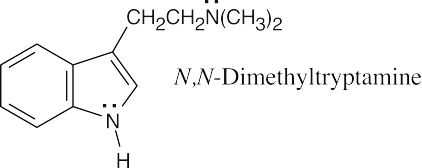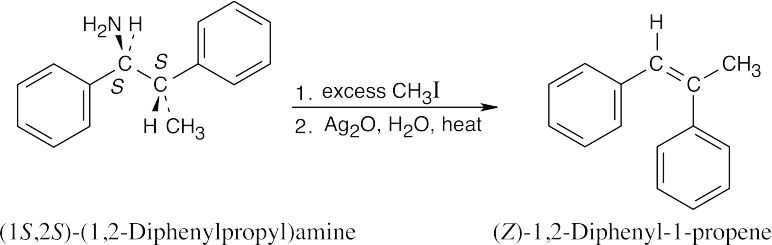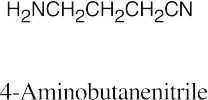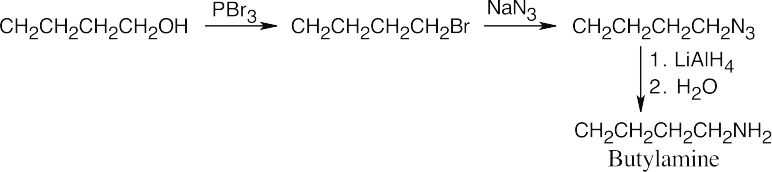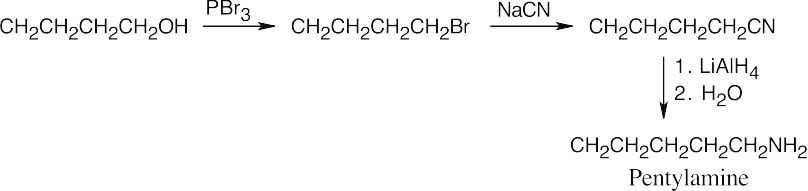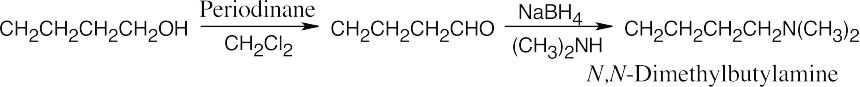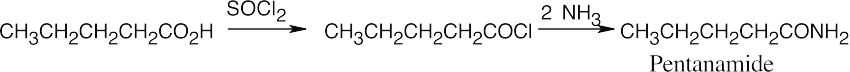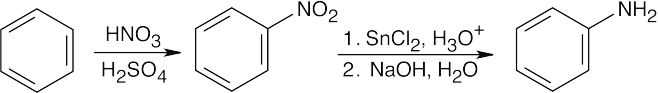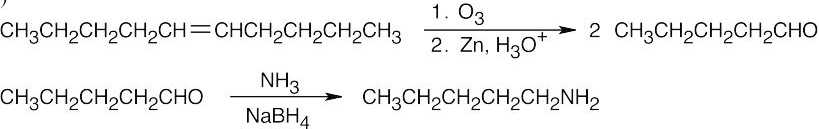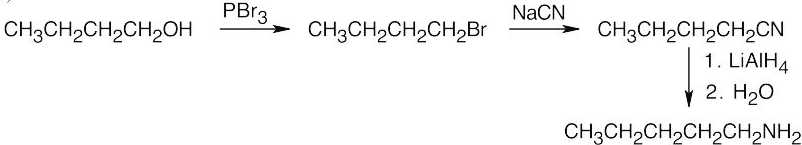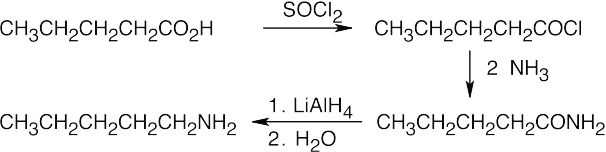12 Chapter 12 Solutions to Problems – Amines and Heterocycles
Chapter 12 – Amines and Heterocycles
Solutions to Problems
| 12.1 | Facts to remember about naming amines:
|
| 12.2 |
|
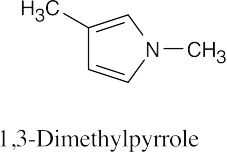
| 12.3 | The numbering of heterocyclic rings is described in Section 12.1.
|
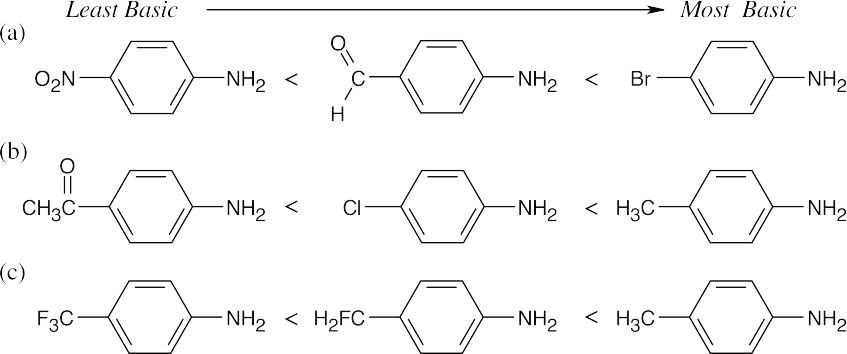
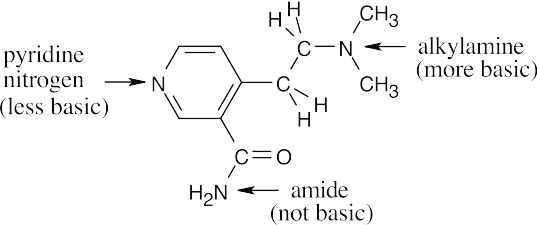
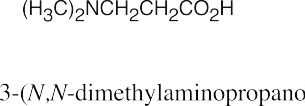
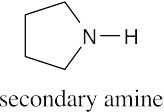
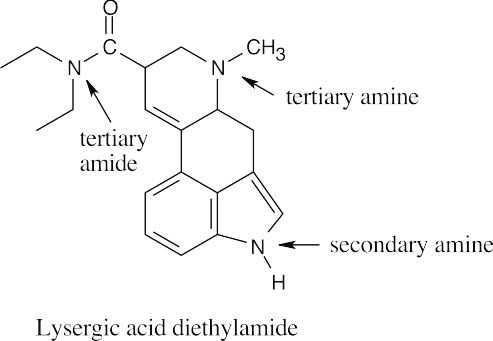
| 12.4 | Amines are less basic than hydroxide but more basic than amides. The pKa values of the conjugate acids of the amines in (c) are shown. The larger the pKa, the stronger the base.
|
| 12.5 |
The stronger base (propylamine) holds a proton more tightly than the weaker base (benzylamine). Thus, the propylammonium ion is less acidic (larger pKa) than the benzylammonium ion (smaller pKa). To calculate pKb: Ka · Kb = 1 x 10–14, pKa + pKb = 14 and pKb = 14 – pKa. |
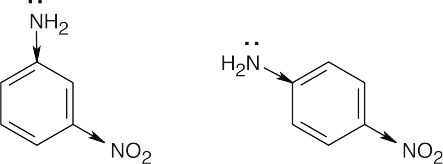
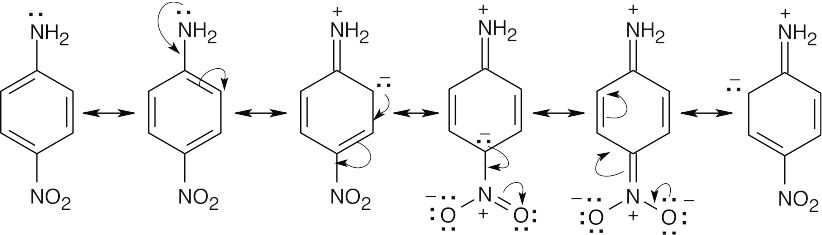
| 12.6 | The basicity order of substituted arylamines is the same as their reactivity order in electrophilic aromatic substitution reactions because, in both cases, electron-withdrawing substituents make the site of reaction more electron-poor and destabilize a positive charge.
|
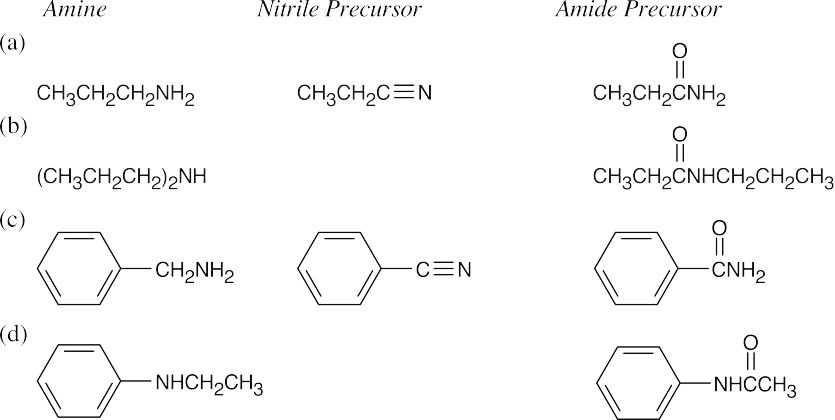
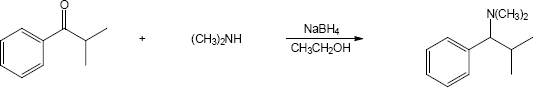
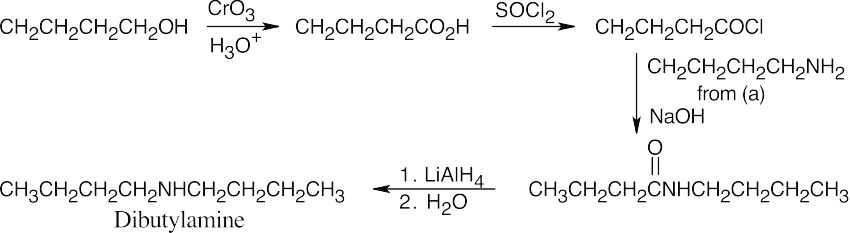
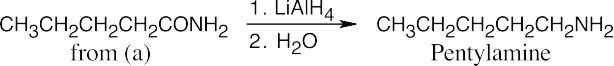
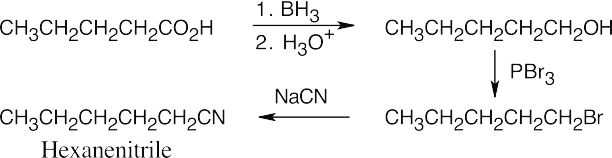
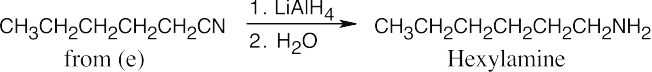
| 12.7 | Amide reduction can be used to synthesize most amines, but nitrile reduction can be used to synthesize only primary amines. Thus, the compounds in (b) and (d) can be synthesized only by amide reduction.
The compounds in parts (b) and (d) can’t be prepared by reduction of a nitrile. |
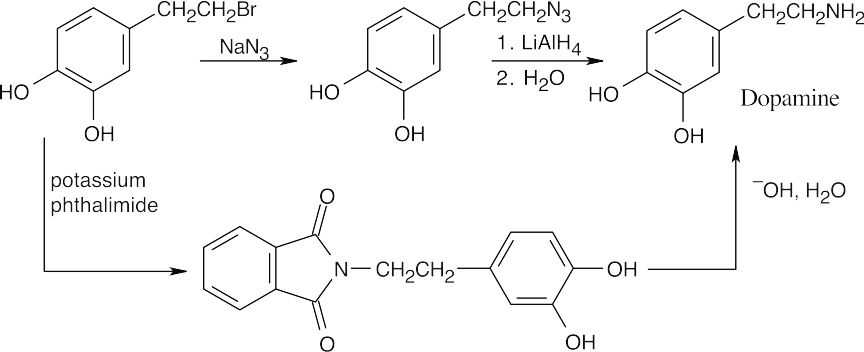
| 12.8 | The upper reaction is the azide synthesis, and the lower reaction is the Gabriel synthesis.
|
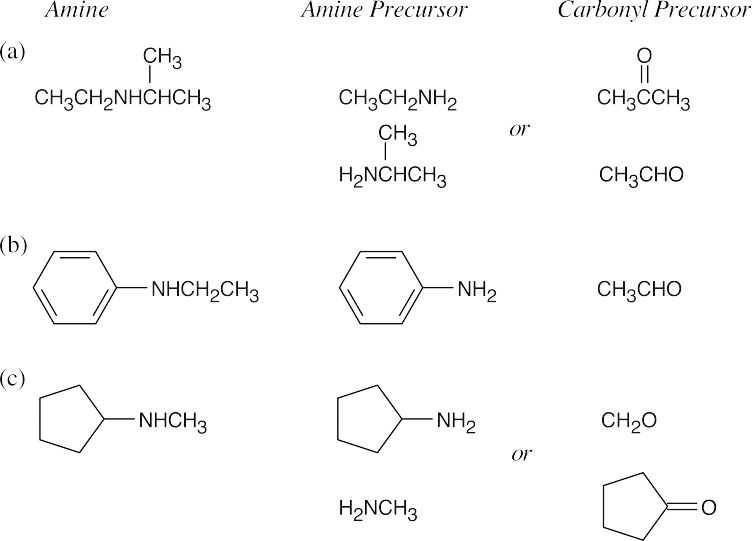
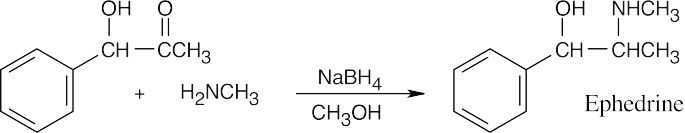
| 12.9 | Look at the target molecule to find the groups bonded to nitrogen. One group comes from the aldehyde/ketone precursor, and the other group comes from the amine precursor. In most cases, two combinations of amine and aldehyde/ketone are possible.
|
| 12.10 |
|
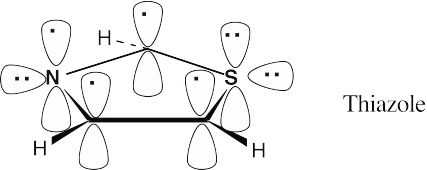
| 12.11 |
Thiazole contains six π electrons. Each carbon contributes one electron, nitrogen contributes one electron, and sulfur contributes two electrons to the ring π system. Both sulfur and nitrogen have lone electron pairs in sp2 orbitals that lie in the plane of the ring. |
| 12.12 |
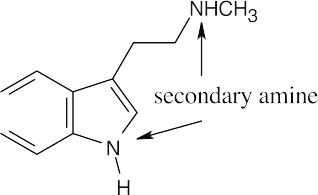
The side chain nitrogen atom of N,N-dimethyltryptamine is more basic than the ring nitrogen atom because its lone electron pair is more available for donation to a Lewis acid. The aromatic nitrogen electron lone pair is part of the ring π electron system. |
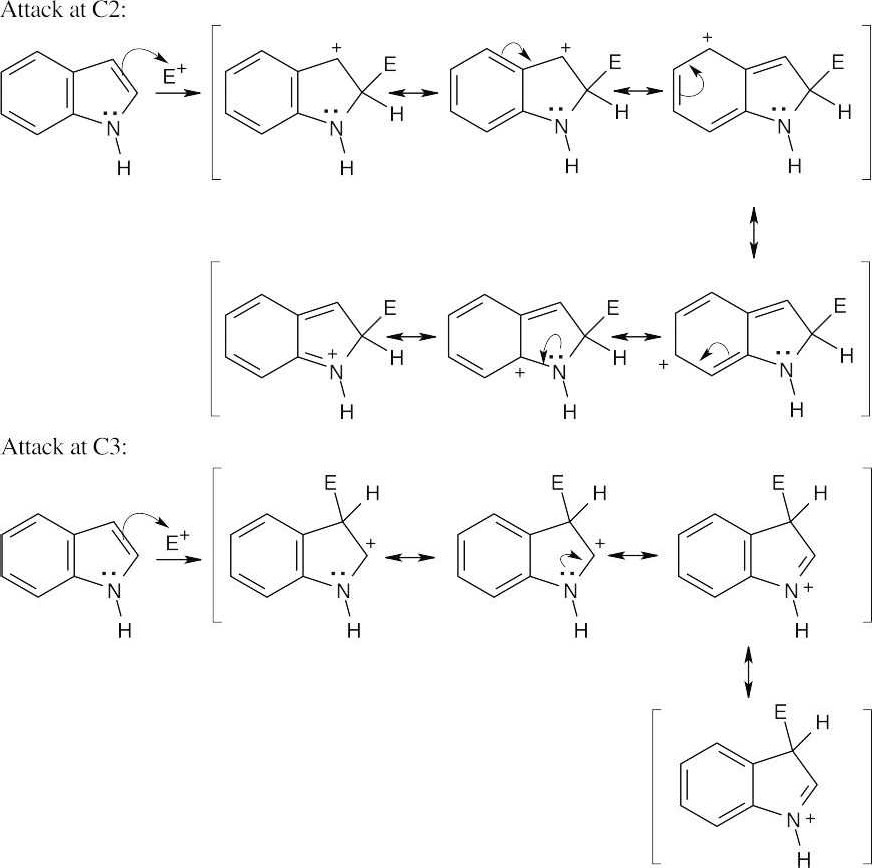
| 12.13 |
Positive charge can be stabilized by the nitrogen lone-pair electrons in reaction at both C2 and C3. In reaction at C2, however, stabilization by nitrogen destroys the aromaticity of the fused benzene ring. Reaction at C3 is therefore favored, even though the cationic intermediate has fewer resonance forms, because the aromaticity of the six-membered-ring is preserved. |
Additional Problems
Visualizing Chemistry
| 12.14 |
|
| 12.15 |
|
| 12.16 |
Hofmann elimination is an E2 elimination, in which the two groups to be eliminated must be 180° apart. The product that results from this elimination geometry is the Z isomer. |
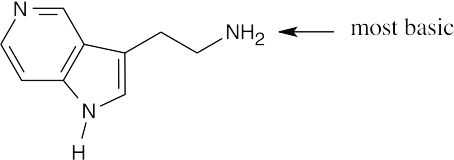
| 12.17 |
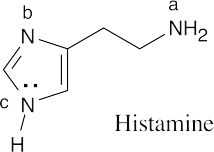
The indicated nitrogen is most basic because it is more electron-rich. The electrons of the other nitrogens are part of the fused-ring π system and are less available for donation to a Lewis acid. |
Mechanism Problems
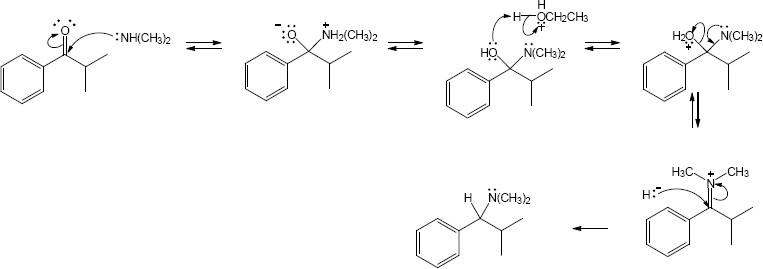
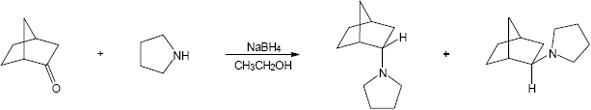
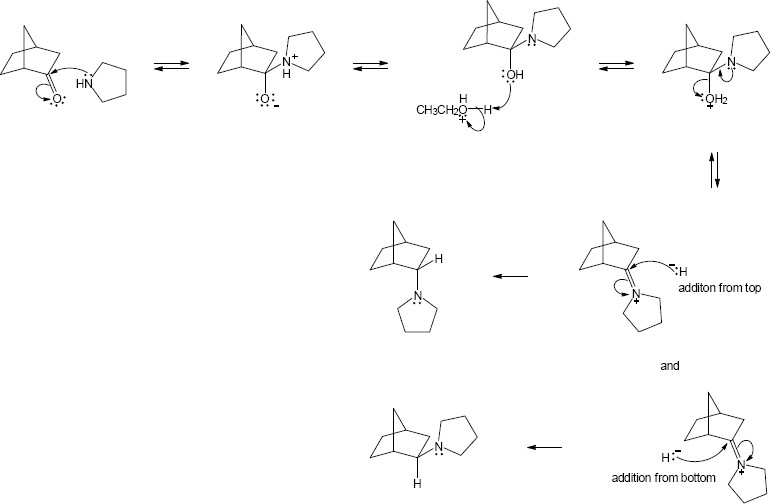
| 12.18 |
|
Naming Amines
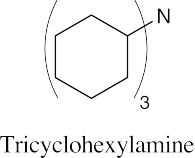
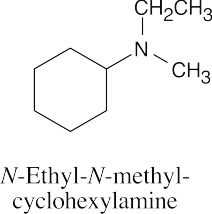
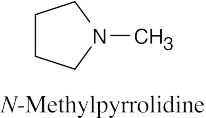
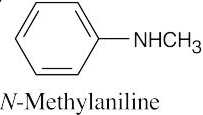
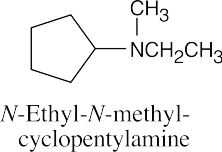
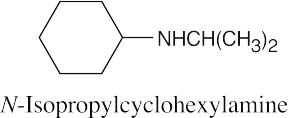
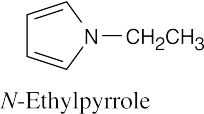
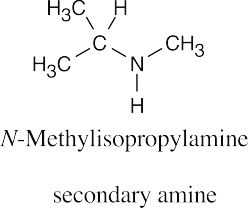
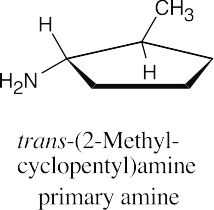
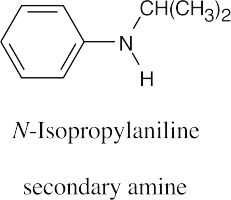
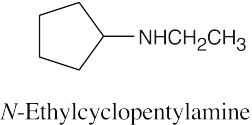
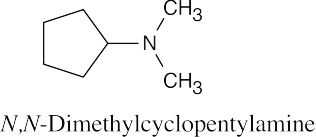
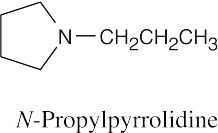
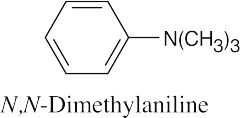
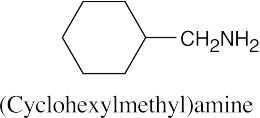
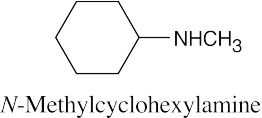
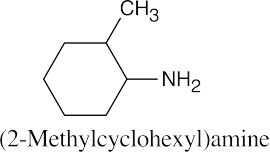
| 12.19 |
|
| 12.20 |
|
| 12.21 |
|
Amine Basicity
| 12.22 | The pyrrole anion, C4H4N:–, is a 6 π electron species that has the same electronic structure as the cyclopentadienyl anion. Both of these anions possess the aromatic stability of 6 π electron systems. |
| 12.23 |
The “a” nitrogen is most basic because its electron pair is most available to Lewis acids. The “c” nitrogen is the least basic because the lone-pair electrons of the pyrrole nitrogen are part of the ring π electron system. |
| 12.24 |
|
Synthesis of Amines
| 12.25 |
|
| 12.26 |
|
| 12.27 |
|
| 12.28 |
|
| 12.29 |
|
General Problems
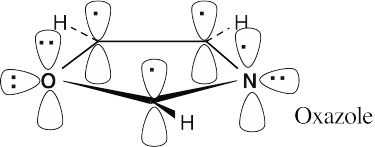
| 12.30 |
Oxazole is an aromatic 6 π electron heterocycle. Two oxygen electrons and one nitrogen electron are in p orbitals that are part of the π electron system of the ring, along with one electron from each carbon. An oxygen lone pair and a nitrogen lone pair are in sp2 orbitals that lie in the plane of the ring. Since the nitrogen lone pair is available for donation to acids, oxazole is more basic than pyrrole. |
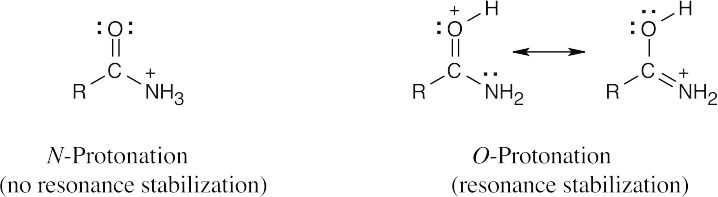
| 12.31 |
Protonation occurs on oxygen because an O-protonated amide is stabilized by resonance. |
| 12.32 |
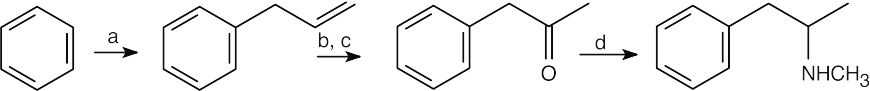
(a) CH2=CHCH2Cl, AlCl3; |
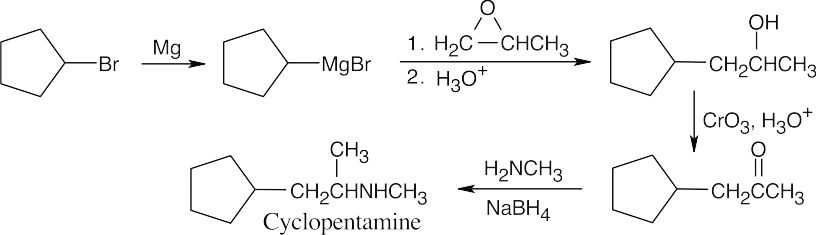
| 12.33 |
The last step of the synthesis is a reductive amination of a ketone that is formed by oxidation of the corresponding alcohol. The alcohol results from the Grignard reaction between cyclopentylmagnesium bromide and propylene oxide. |
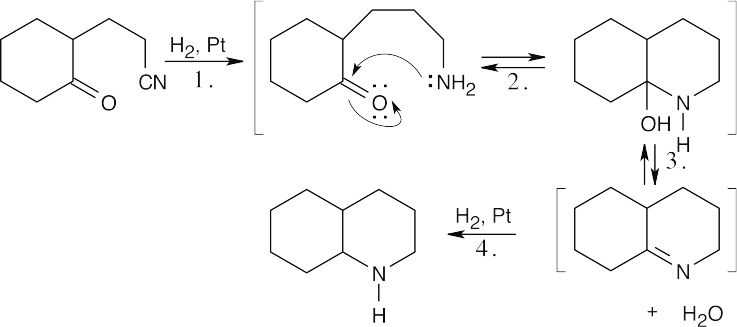
| 12.34 | The formula C9H17N indicates two degrees of unsaturation in the product. Both are probably due to rings since the product results from catalytic reduction.
Step 1: Reduction of nitrile Step 2: Nucleophilic addition. Step 3: Dehydration. Step 4: Reduction of double bond. |
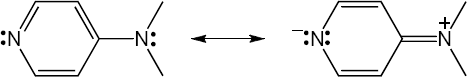
| 12.35 | Note: Although there are several resonance forms, with the exception of the completely neutral form, the most stable one is the charge separated form with a negative charge on the pyridine nitrogen. The increased electron density on the pyridine nitrogen increases its ability to react as a nucleophile.
|