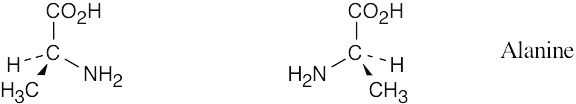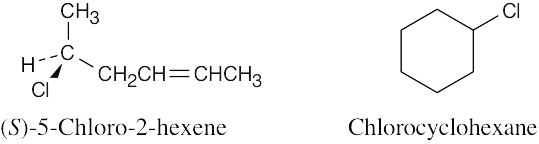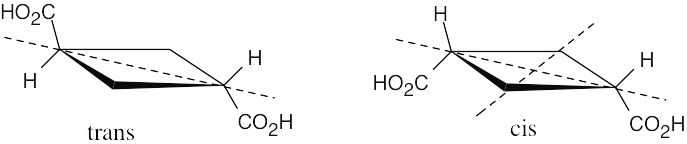3 Chapter 3 Solutions to Problems – Stereochemistry at Tetrahedral Centers
Chapter 3 – Stereochemistry at Tetrahedral Centers
Solutions to Problems
| 3.1 |
Objects having a plane of symmetry are achiral. Chiral: screw, shoe. Achiral: soda can, screwdriver. |
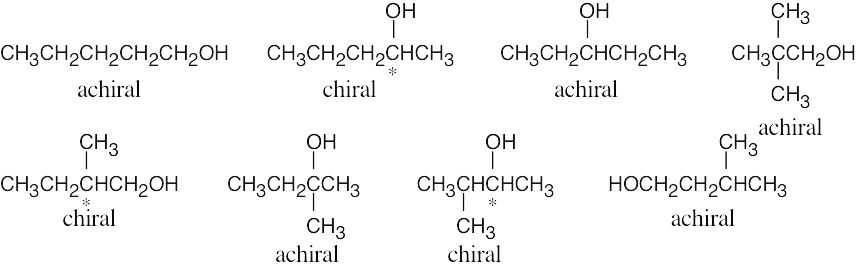
| 3.2 | Use the following rules to locate centers that are not chirality centers, then examine the remaining centers to find a carbon with four different groups attached. | |
| 1. | All –CH3 and –CX3 carbons are not chirality centers. | |
| 2. | All –CH2– and –CX2– carbons are not chirality centers. | |
| 3. | All 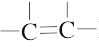 and and 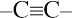 carbons are not chirality centers. By rule 3, all aromatic ring carbons are not chirality centers. carbons are not chirality centers. By rule 3, all aromatic ring carbons are not chirality centers. |
|
| (a) | 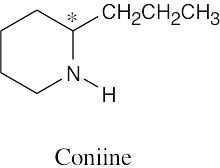 |
|
| (b) | 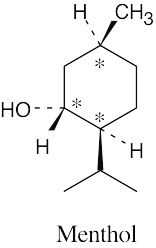 |
|
| (c) |  |
|
| 3.3 | Refer to Problem 3.2 if you need help.
|
| 3.4 | (a) | 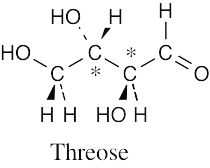 |
| (b) | 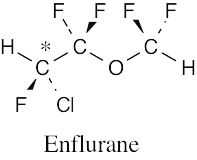 |
| 3.5 | By convention, a (–) rotation indicates rotation to the left, and thus cocaine is levorotatory. |
| 3.6 | Review the sequence rules presented in Section 3.5. A summary: | |||||||||||||||||||||||||||||
| Rule 1: | An atom with a higher atomic number has priority over an atom with a lower atomic number. | |||||||||||||||||||||||||||||
| Rule 2: | If a decision can’t be reached by using Rule 1, look at the second, third, or fourth atom away from the double-bond carbon until a decision can be made. | |||||||||||||||||||||||||||||
| Rule 3: | Multiple-bonded atoms are equivalent to the same number of single-bonded atoms. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
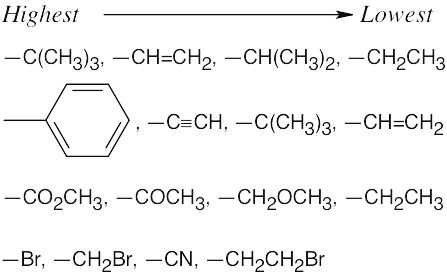
| 3.7 | Use the sequence rules in Section 3.5. | |
| (a) |
By Rule 1, –H is of lowest priority, and –OH is of highest priority. By Rule 2, –CH2CH2OH is of higher priority than –CH2CH3. Highest → Lowest –OH, –CH2CH2OH, –CH2CH3, –H |
|
| (b) |
By Rule 3, –CO2H is considered as Highest → Lowest |
|
| (b) | –OH, –CO2CH3, –CO2H, –CH2OH | |
| (c) | –NH2, –CN, –CH2NHCH3, –CH2NH2 | |
| (d) | –SSCH3, –SH, –CH2SCH3, –CH3 | |
| 3.8 | All stereochemistry problems are easier if you use models. Part (a) will be solved by two methods – with models and without models. | |
| (a) |
With models: Build a model of (a). Orient the model so that group 4 is pointing to the rear. Note the direction of rotation of arrows that go from group 1 to group 2 to group. The arrows point counterclockwise, and the configuration is S. Without models: Imagine yourself looking at the molecule, with the group of lowest priority pointing to the back. Your viewpoint would be at the upper right of the molecule, and you would see group 1 on the left, group 3 on the right and group 2 at the bottom. The arrow of rotation travels counterclockwise, and the configuration is S. |
|
| (a) | 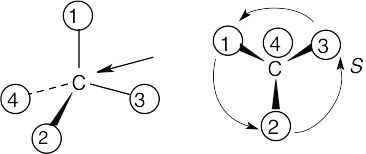 |
|
| (b) | 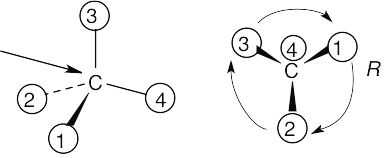 |
|
| (c) | 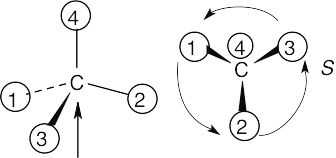 |
|
| 3.9 | Step 1: |
For each chirality center, rank substituents by the Cahn–Ingold–Prelog system; give the number 4 to the lowest priority substituent. For part (a):
|
|||||||||||
| Step 2: | As in the previous problem, orient yourself so that you are 180° from the lowest priority group (indicated by the arrow in the drawing). From that viewpoint, draw the molecule as it looks when you face it. Draw the arrow that travels from group 1 to group 2 to group 3, and note its direction of rotation. The molecule in (a) has S configuration. | ||||||||||||
| (a) | 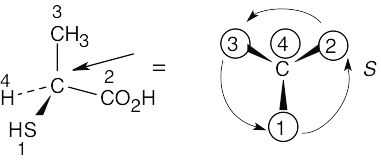 |
||||||||||||
| (b) | 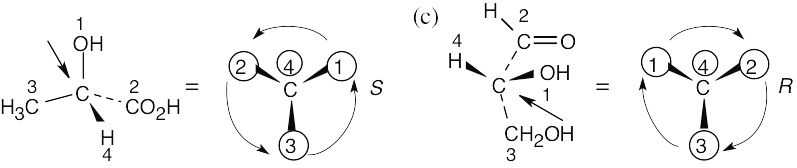
In (b), the observer is behind the page, looking out and down toward the right. In (c), the observer is behind the page looking out and up to the left. |
||||||||||||
| 3.10 | 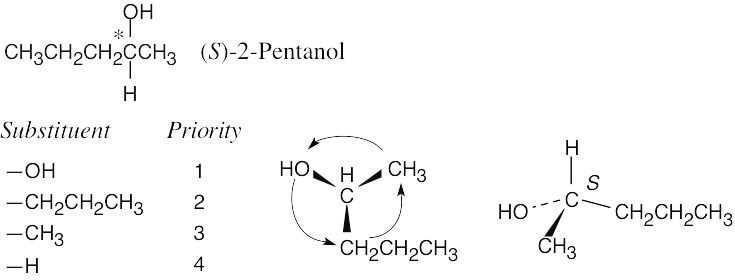 |
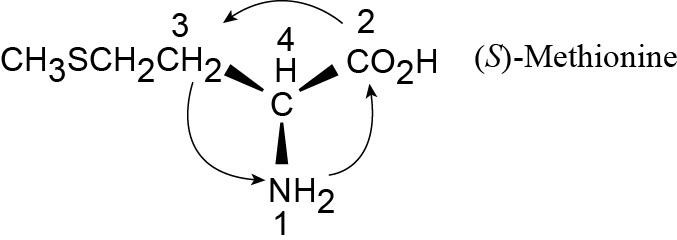
| 3.11 |
Fortunately, methionine is shown in the correct orientation.
|
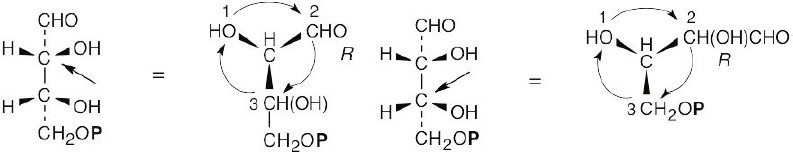
| 3.12 |
For (a): (Note: the phosphate group is represented as P.)
(a) R,R (b) S,R (c) R,S (d) S,S a, d are enantiomers and are diastereomeric with b, c. b, c are enantiomers and are diastereomeric with a, d. Structure (a) is D-erythrose 4-phosphate, structure (d) is its enantiomer, and structures (b) and (c) are its diastereomers. |
| 3.13 | 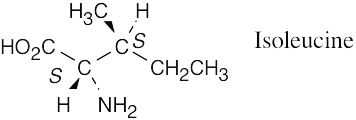 |
| 3.14 | To decide if a structure represents a meso compound, try to locate a plane of symmetry that divides the molecule into two halves that are mirror images. Molecular models are always helpful. | |
| (a) | 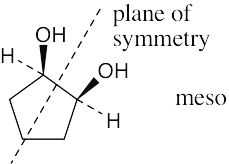 |
|
| (b) | and (c) are not meso structures | |
| (d) | 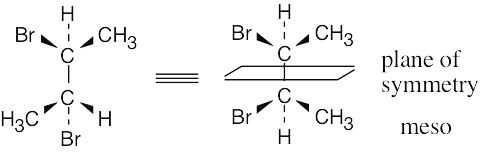 |
|
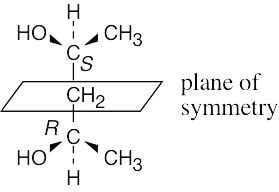
| 3.15 | For a molecule to exist as a meso form, it must possess a plane of symmetry. 2,3- Butanediol can exist as a pair of enantiomers or as a meso compound, depending on the configurations at carbons 2 and 3. | |
| (a) | 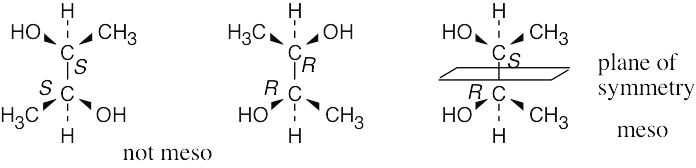 |
|
| (b) | 2,3-Pentanediol has no symmetry plane and thus can’t exist in a meso form. | |
| (c) |
2,4-Pentanediol can exist in a meso form.
2,4-Pentanediol can also exist as a pair of enantiomers (2R,4R) and (2S,4S) that are not meso compounds. |
|
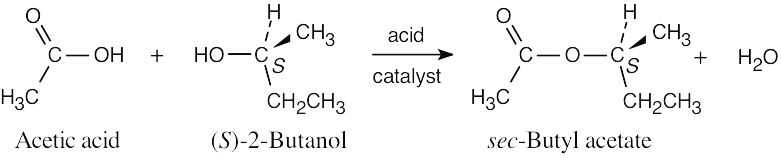
| 3.16 |
The product is the pure S-ester. No new chirality centers are formed during the reaction, and the configuration at the chirality center of (S)-2-butanol is unchanged.
|
| 3.17 | (a) |
These two compounds are constitutional isomers (skeletal isomers).
|
| (b) | The two dibromopentane stereoisomers are diastereomers. |
Additional Problems
Visualizing Chemistry
| 3.18 | Structures (a), (b), and (d) are identical (R enantiomer), and (c) represents the S enantiomer. |
| 3.19 | (a) | 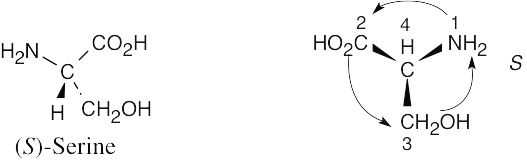 |
| (b) | 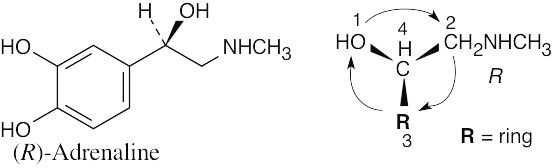 |
| 3.20 | Locate the plane of symmetry that identifies the structure as a meso compound. | |
| (a) | 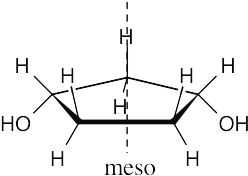 |
|
| (b) | 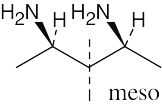 |
|
| (c) | 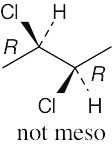 |
|
| 3.21 | 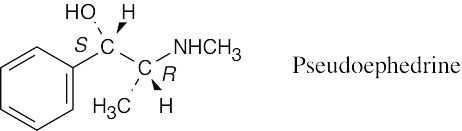 |
| 3.22 | (a) | 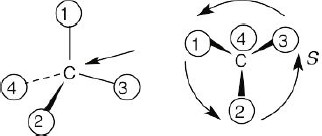 |
| (b) | 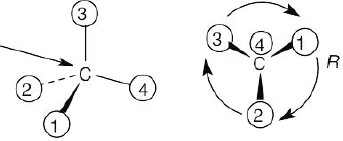 |
|
| (c) | 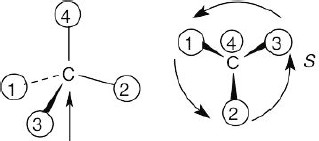 |
Chirality and Optical Activity
| 3.23 |
Chiral: (d) golf club, (e) spiral staircase Achiral: (a) basketball, (b) fork, (c) wine glass, (f) snowflake |
| 3.24 | (a) | 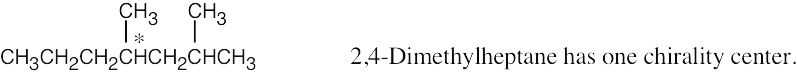 |
| (b) | 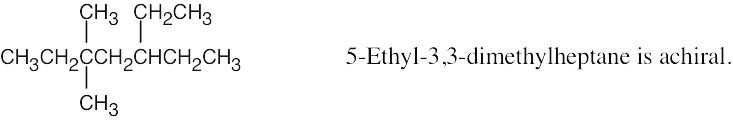 |
|
| (c) | 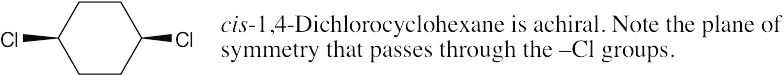 |
| 3.25 | (a) | 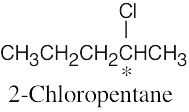 |
| (b) | 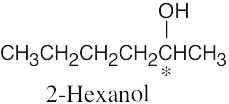 |
|
| (c) | 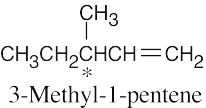 |
|
| (d) | 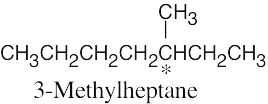 |
| 3.26 |
|
| 3.27 | (a) | 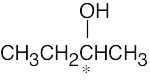 |
| (b) | 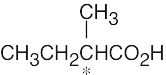 |
|
| (c) | 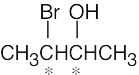 |
|
| (d) | 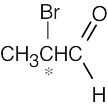 |
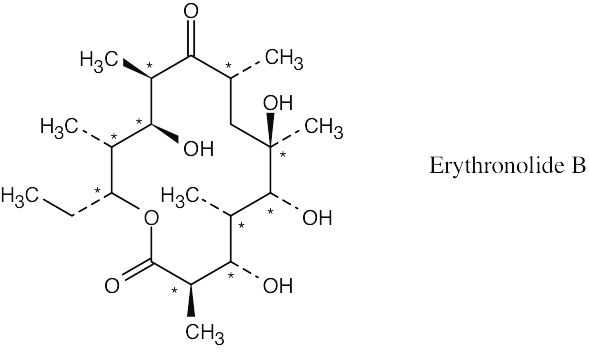
| 3.28 |
Erythronolide B has ten chirality centers.
|
Assigning Configuration to Chirality Centers
| 3.29 | Identical molecules: (b) (S enantiomer), (c) (R enantiomer), (d) (S enantiomer); Pair of enantiomers: (a) |
| 3.30 | The specific rotation of (2R,3R)-dichloropentane is equal in magnitude and opposite in sign to the specific rotation of (2S,3S)-dichloropentane because the compounds are enantiomers. There is no predictable relationship between the specific rotations of the (2R,3S) and (2R,3R) isomers because they are diastereomers. |
| 3.31-3.32 | 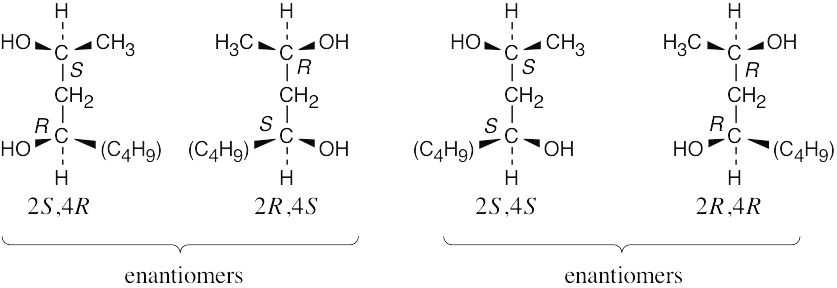
The (2R,4S) stereoisomer is the enantiomer of the (2S,4R) stereoisomer. The (2S,4S) and (2R,4R) stereoisomers are diastereomers of the (2S,4R) stereoisomer. |
| 3.33 | (a) | 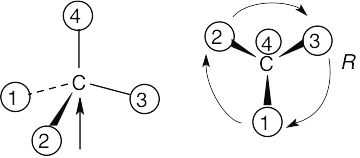 |
| (b) | 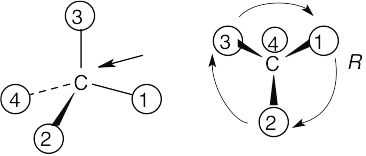 |
|
| (c) | 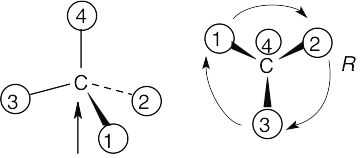 |
| 3.34 |
|
|
| (a) | ||
| (b) | ||
| (c) | ||
| (d) |
| 3.35 | (a) | 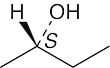 |
| (b) | 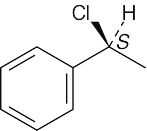 |
|
| (c) | 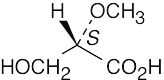 |
| 3.36 | (a) | 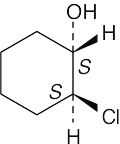 |
| (b) | 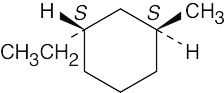 |
|
| (c) | 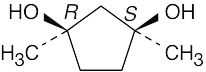 |
| 3.37 | (a) | 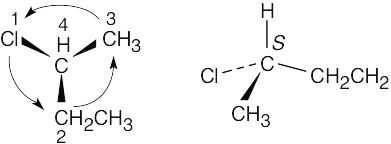 |
| (b) | 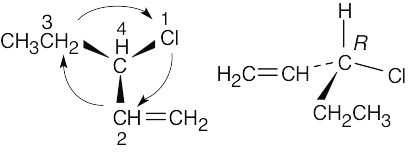 |
| 3.38 | (a) | 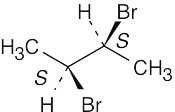 |
| (b) | 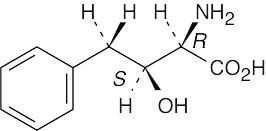 |
| 3.39 | 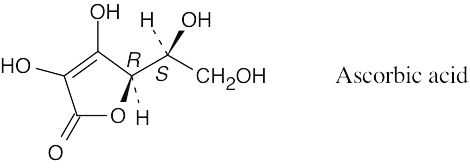 |
| 3.40 | (a) | 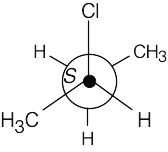 |
| (b) | 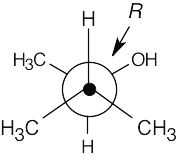 |
| 3.41 | 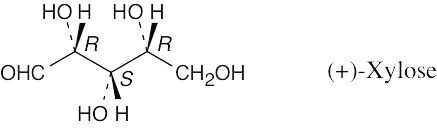 |
Meso Compounds
| 3.42 | (a) | 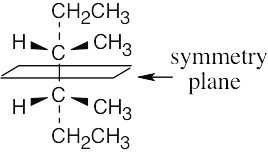 |
| (b) | 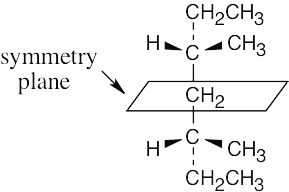 |
|
| (c) | 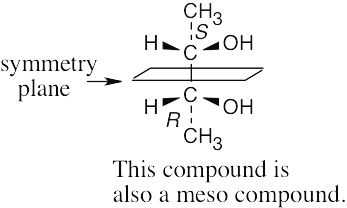 |
| 3.43 | (a)-(c) | 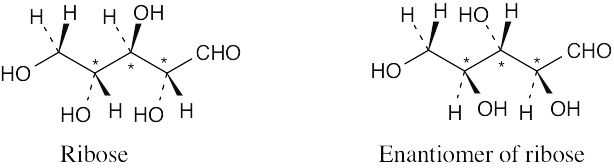
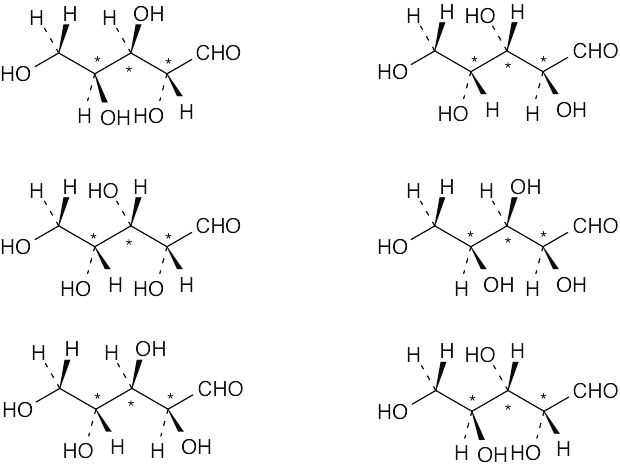
Ribose has three chirality centers, which give rise to eight (23) stereoisomers. |
| (d) | Ribose has six diastereomers.
|
| 3.44 |
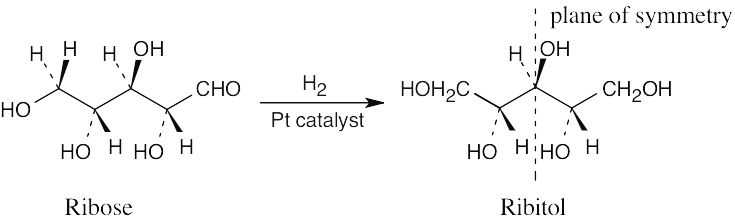
Ribitol is an optically inactive meso compound. Catalytic hydrogenation converts the aldehyde functional group into a hydroxyl group and makes the two halves of ribitol mirror images of each other.
|
General Problems
| 3.45 |
|
| 3.46 | (a) | 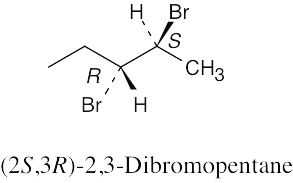 |
| (b) | 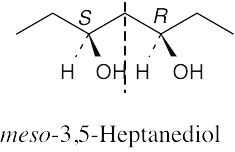 |
| 3.47 | 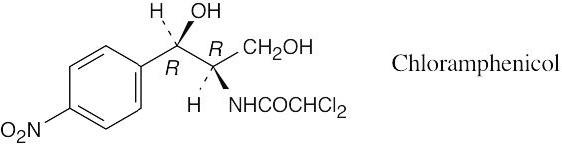 |
| 3.48 |
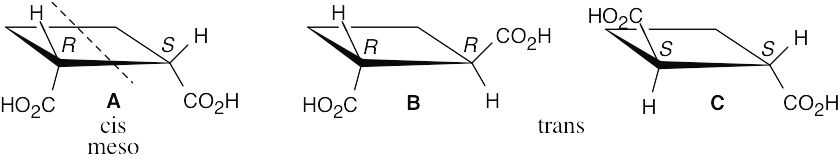
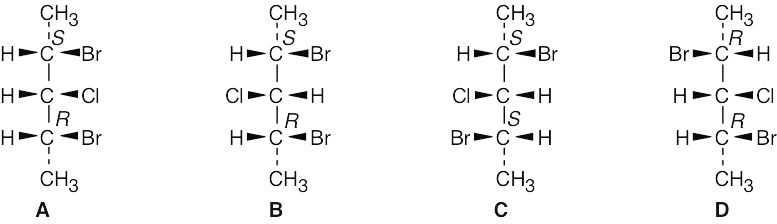
There are four stereoisomers of 2,4-dibromo-3-chloropentane. C and D are enantiomers and are optically active. A and B are optically inactive meso compounds and are diastereomers.
|
| 3.49 | 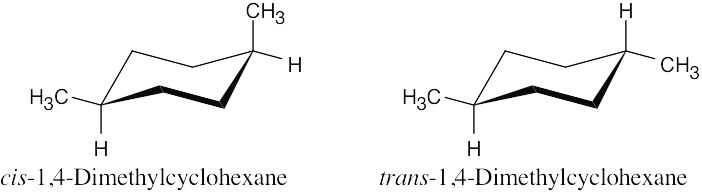 |
|
| (a) | There is only one stereoisomer of each of the 1,4-dimethylcyclohexanes. | |
| (b) | Neither 1,4-dimethylcyclohexane is chiral. | |
| (c) | The two 1,4-dimethylcyclohexanes are diastereomers. | |
| 3.50 | 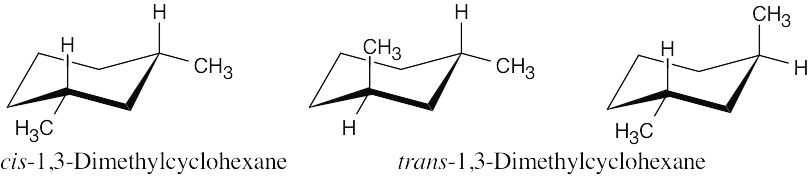 |
|
| (a) | There is one stereoisomer of cis-1,3-dimethylcyclohexane, and there are two stereoisomers of trans-1,3-dimethylcyclohexane. | |
| (b) | cis-1,3-Dimethylcyclohexane is an achiral meso compound; trans-1,3- dimethylcyclohexane exists as a pair of chiral enantiomers. | |
| (c) | The two trans stereoisomers are enantiomers, and both are diastereomers of the cis stereoisomer. | |