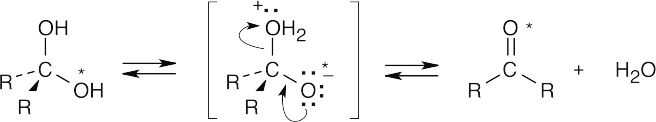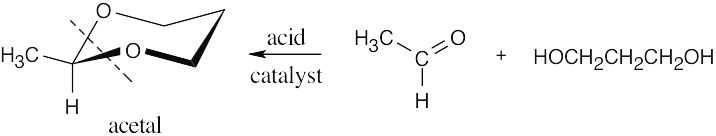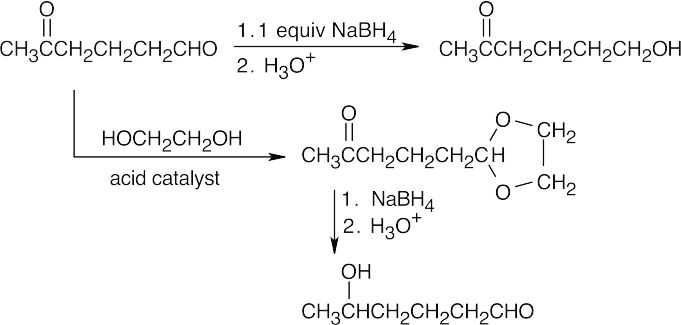10 Chapter 10 Solutions to Problems – Aldehydes and Ketones: Nucleophilic Addition Reactions
Chapter 10 – Aldehydes and Ketones: Nucleophilic Addition Reactions
Solutions to Problems
| 10.1 | Remember that the principal chain must contain the aldehyde or ketone group and that an aldehyde group occurs only at the end of a chain. The aldehyde carbon is carbon 1 in an acyclic compound, and the suffix –carbaldehyde is used when the aldehyde group is attached to a ring. | |||
| (a) | 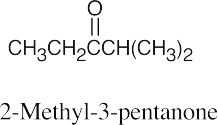 |
(b) | 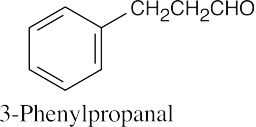 |
|
| (c) | 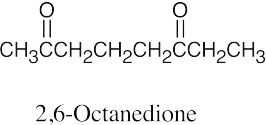 |
(d) | 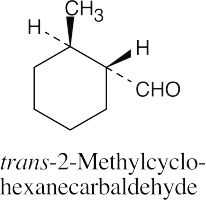 |
|
| (e) | 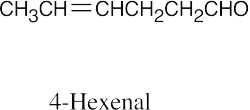 |
(f) | 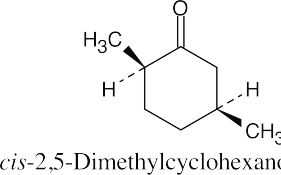 |
|
| 10.2 | (a) | 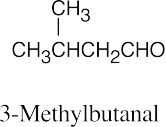 |
(b) | 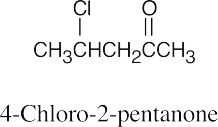 |
| (c) | 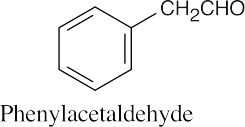 |
(d) | 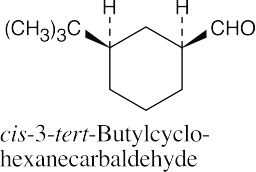 |
|
| (e) | 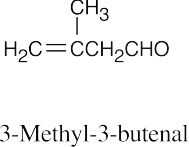 |
(f) | 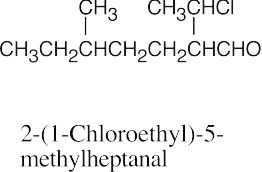 |
| 10.3 | We have seen the first two methods of aldehyde preparation in earlier chapters. | |
| (a) | 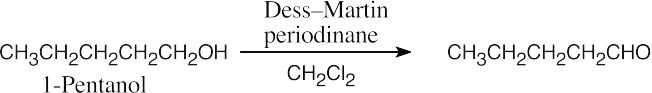 |
|
| (b) | 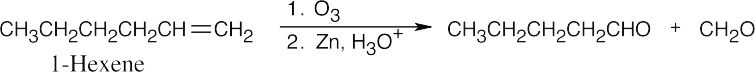 |
|
| (c) | 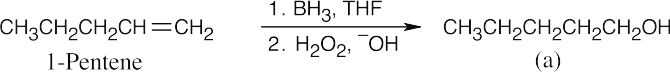 |
|
| 10.4 | All of these methods are familiar. | |
| (a) | 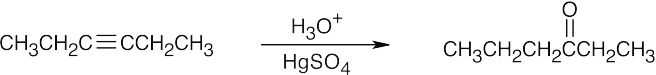 |
|
| (b) | 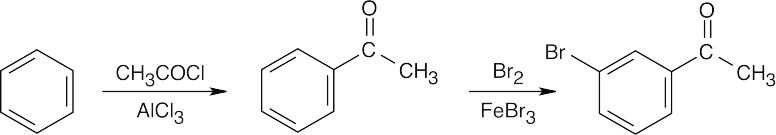 |
|
| (c) | 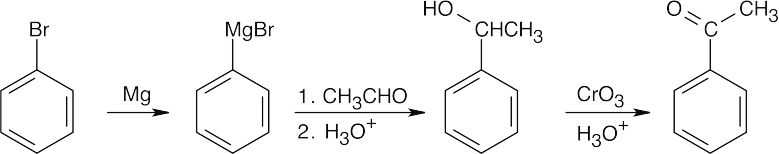 |
|
| 10.5 | 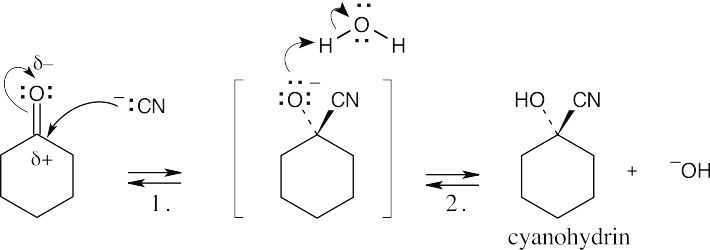 |
|
| Step 1: | Cyanide anion adds to the positively polarized carbonyl carbon to form a tetrahedral intermediate. | |
| Step 2: | This intermediate is protonated to yield the cyanohydrin. | |
| 10.6 | 
The electron-withdrawing nitro group makes the aldehyde carbon of p-nitrobenzaldehyde more electron-poor (more electrophilic) and more reactive toward nucleophiles than the aldehyde carbon of p–methoxybenzaldehyde. |
| 10.7 | 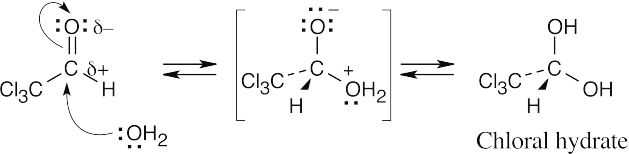 |
| 10.8 | 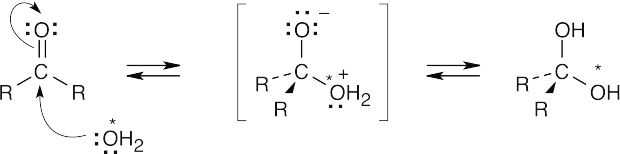
The above mechanism is similar to other nucleophilic addition mechanisms we have studied. Since all steps are reversible, we can write the above mechanism in reverse to show how labeled oxygen is incorporated into an aldehyde or ketone.
This exchange is very slow in water but proceeds more rapidly when either acid or base is present. |
| 10.9 | 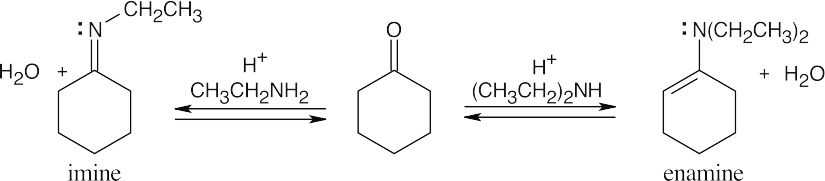
Reaction of a ketone or aldehyde with a primary amine yields an imine, in which C=O has been replaced by C=NR. Reaction of a ketone or aldehyde with a secondary amine yields an enamine, in which C=O has been replaced by C–NR2, and the double bond has moved. |
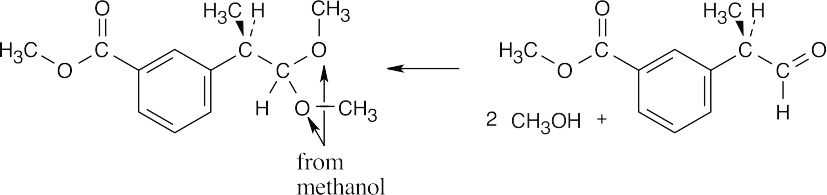
| 10.10 | Locate the two identical –OR groups to identify the alcohol that was used to form the acetal. (The illustrated acetal was formed from methanol.) Replace these two –OR groups by =O to find the carbonyl compound.
|
Additional Problems
Visualizing Chemistry
| 10.11 | It helps to know that all of these substances were prepared from aldehydes or ketones. Look for familiar groupings of atoms to identify the starting materials. | |
| (a) | Notice that the substance pictured is a cyclic acetal. The starting materials were a diol (because cyclic acetals are prepared from diols) and an aldehyde (because an –H is bonded to the acetal carbon). Replace the two –OR groups with =O to identify the aldehyde starting material (acetaldehyde).
|
|
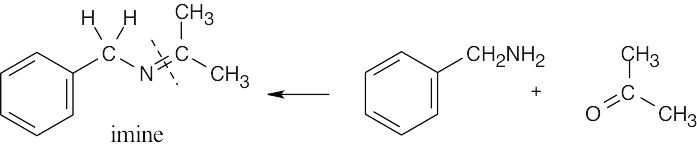
| (b) | We know that the product is an imine because it contains a carbon-nitrogen double bond. The carbon that is part of the C=N bond came from a ketone, and the nitrogen came from a primary amine.
|
|
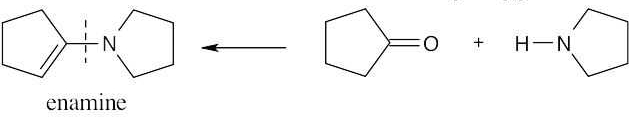
| (c) | The product is an enamine, formed from a ketone and a secondary amine. Nitrogen is bonded to the carbon that once bore the carbonyl oxygen.
|
|
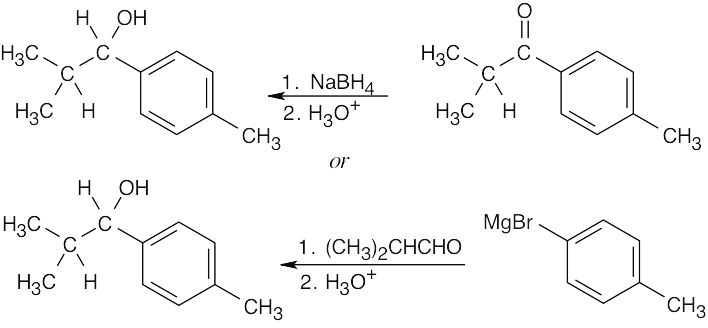
| (d) | The secondary alcohol product might have been formed by either of two routes – by reduction of a ketone or by Grignard addition to an aldehyde.
|
|
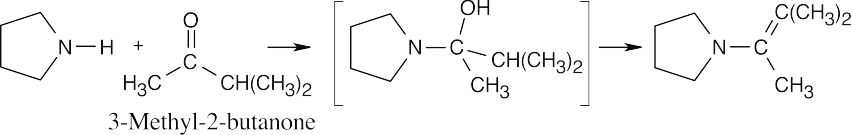
| 10.12 | The intermediate results from the addition of an amine to a ketone. The product is an enamine because the amine nitrogen in the carbinolamine intermediate comes from a secondary amine.
|
Mechanism Problems
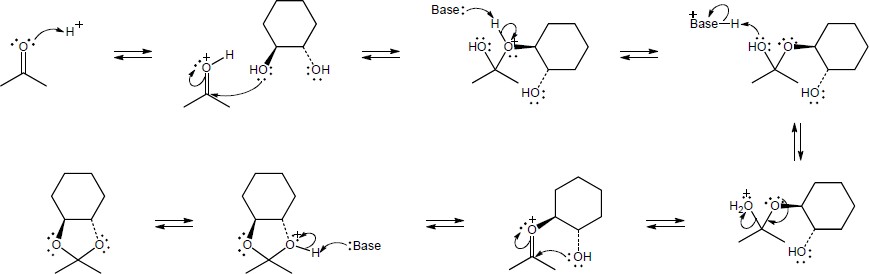
| 10.13 | Note: Each reaction is an example of acetal formation and follows the same pattern: 1) protonation of the carbonyl oxygen; 2) nucleophilic addition of an alcohol oxygen; 3) removal of a proton from the oxygen now bearing the positive charge to give a hemiacetal intermediate. This is often accomplished with the solvent. In the problems where no solvent is given, a general base has been used for these solutions; 4) protonation of the hemiacetal OH converting it to water, a good leaving group; 5) loss of water to form a highly electrophilic oxonium ion; 6) nucleophilic addition of a second alcohol oxygen to the carbon adjacent to the oxonium ion; 7) deprotonation to yield the acetal product. | |
| (a) | 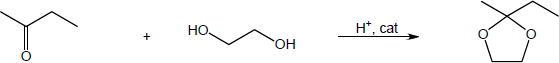
Mechanism:
|
|
| (b) | 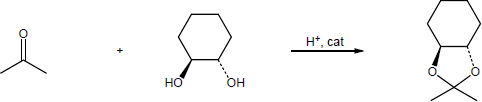
Mechanism:
|
|
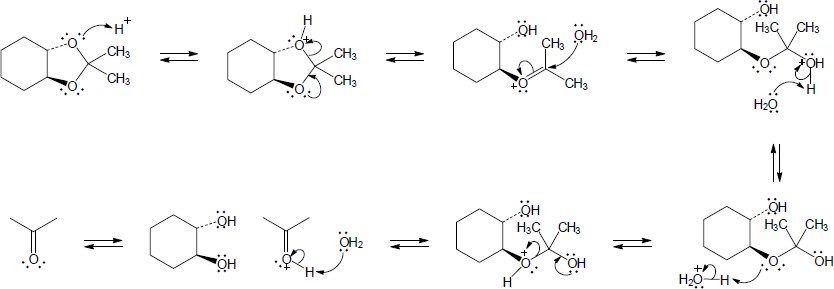
| 10.14 | Note: Each mechanism is the acid catalyzed hydrolysis of an acetal and follows the same steps. Fundamentally, acetal hydrolysis is the mechanism for acetal formation in reverse. The steps are: 1) Protonation of one of the acetal oxygens; 2) Loss of an alcohol molecule with simultaneous formation of an oxonium ion; 3) Nucleophilic addition of water to the carbon that is double bonded to the oxonium ion; 4) Deprotonation of the oxygen with the positive charge to give a hemiacetal intermediate; 5) Protonation of the ether oxygen of the hemiacetal; 6) Loss of a second alcohol molecule with simultaneous formation of carbon-oxygen double double bond; 7) Deprotonation of the carbonyl oxygen to give the carbonyl. | |
| (a) | 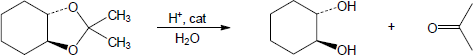
Mechanism:
|
|
| (b) | 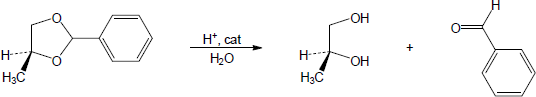
Mechanism:
|
|
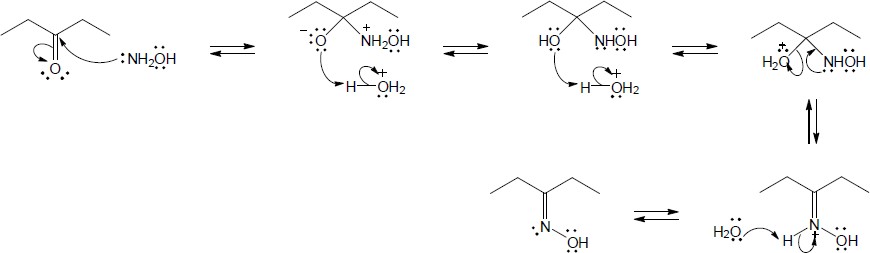
| 10.15 | Note: Each mechanism involves the following steps for iminium ion formation: 1) Nucleophilic addition of the amine nitrogen to the carbonyl carbon to form a dipolar tetrahedral intermediate; 2) Proton transfer to yield a neutral carbonolamine; 3) Protonation of the OH group of the carbonolamine to convert it into a water, a good leaving group; 4) Loss of water through use of the lone pair of electrons on the nitrogen of the carbinolamine to form an iminium ion. At this point, the mechanism differs: if the amine is primary, the iminium ion is deprotonated to yield an imine; if the amine is secondary, an alpha hydrogen is removed in an E2-like process to yield an enamine. | |
| (a) | 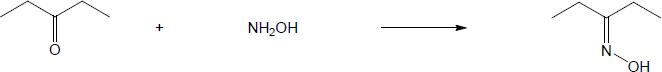
Mechanism:
|
|
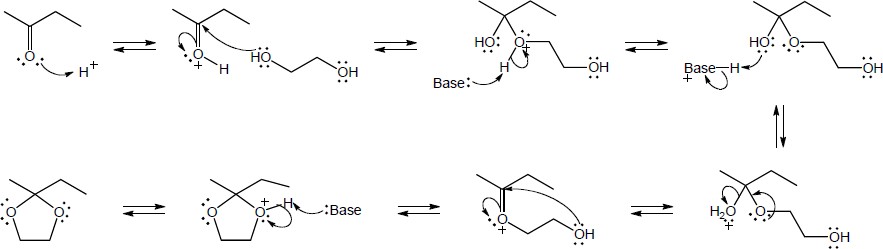
| 10.16 | The same series of steps used to form an acetal is followed in this mechanism.
|
|
| Step 1: | Protonation. | |
| Step 2: | Addition of RSH. | |
| Step 3: | Loss of proton. | |
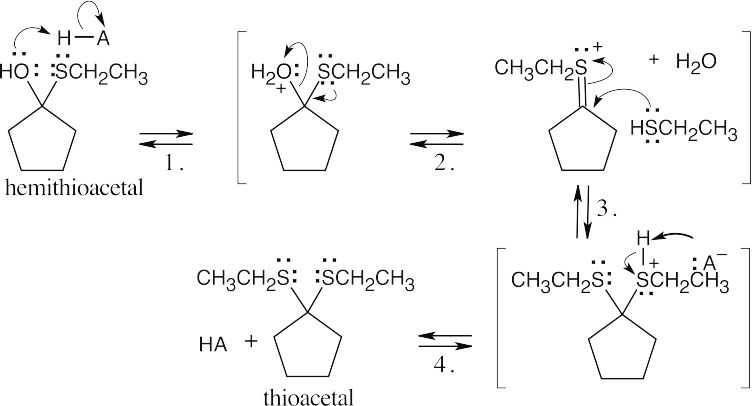 |
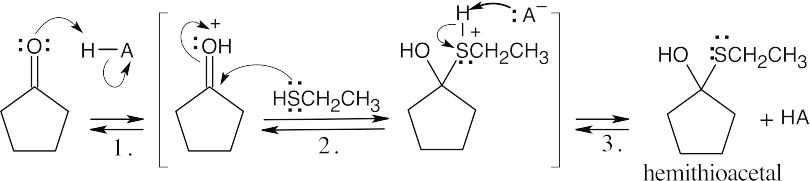
||
| Step 1: | Protonation. | |
| Step 2: | Loss of water. | |
| Step 3: | Addition of RSH. | |
| Step 4: |
Loss of proton. | |
Naming Aldehydes and Ketones
| 10.17 | (a) | 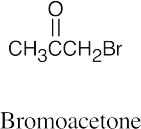 |
(b) | 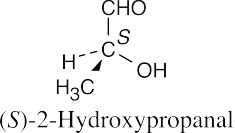 |
(c) | 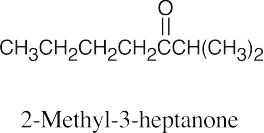 |
| (d) | 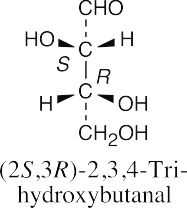 |
(e) | 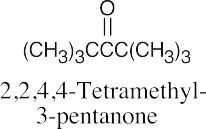 |
(f) | 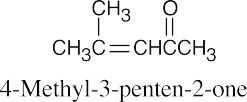 |
|
| (g) | 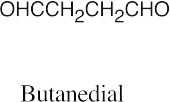 |
(h) | 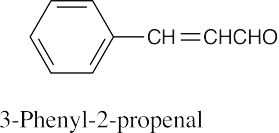 |
(i) | 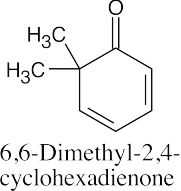 |
|
| (j) | 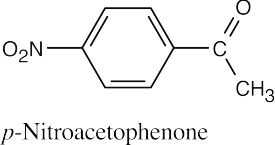 |
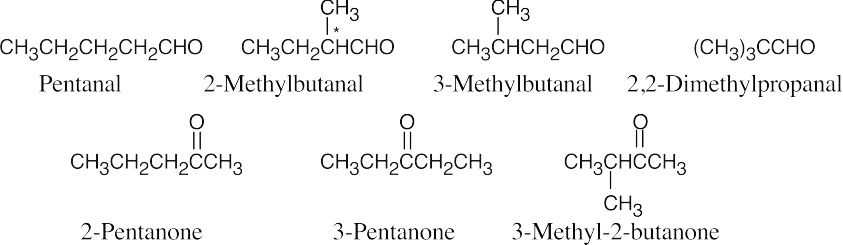
| 10.18 | Only 2-methylbutanal is chiral.
|
| 10.19 | (a) | 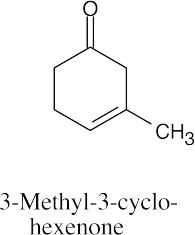 |
(b) | 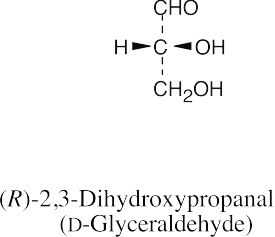 |
(c) |
|
| (d) | 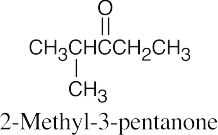 |
(e) | 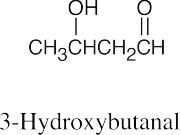 |
(f) | 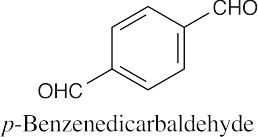 |
| 10.20 | (a) | 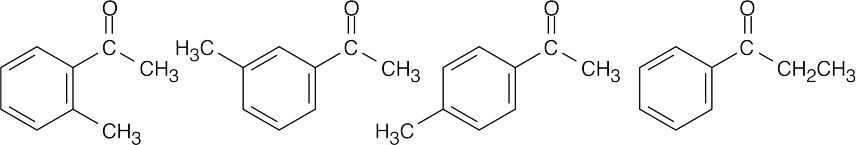 |
| (b) | 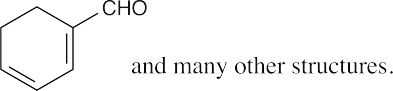 |
Reactions of Aldehydes and Ketones
| 10.21 | Reactions of phenylacetaldehyde: | |||||
| (a) | 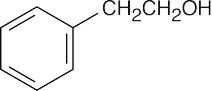 |
(b) | 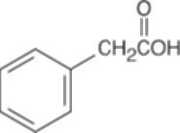 |
(c) | 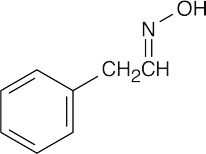 |
|
| (d) | 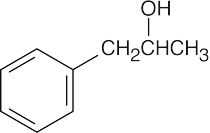 |
(e) | 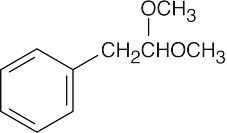 |
(f) | 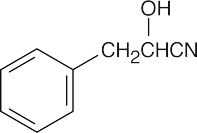 |
|
| Reactions of acetophenone: | ||||||
| (a) | 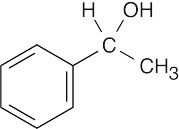 |
(b) | No reaction | (c) | 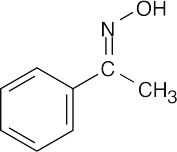 |
|
| (d) | 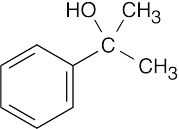 |
(e) | 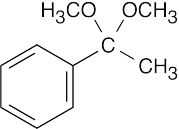 |
(f) | 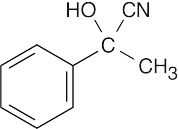 |
|
| 10.22 | Remember from Chapter 9:
Primary alcohols are formed from formaldehyde + Grignard reagent. Secondary alcohols are formed from an aldehyde + Grignard reagent. Tertiary alcohols are formed from a ketone (or an ester) + Grignard reagent. |
|||||
| Aldehyde/Ketone Grignard reagent Product (after acidic workup) | ||||||
| (a) | 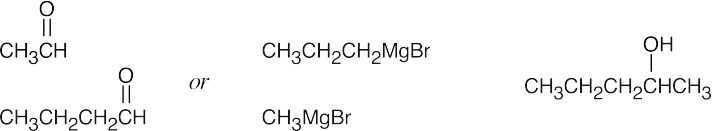 |
|||||
| (b) | 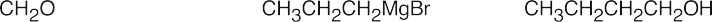 |
|||||
| (c) | 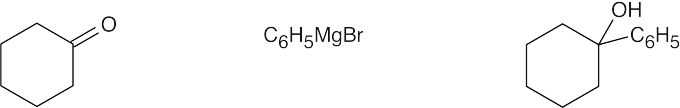 |
|||||
| (d) | 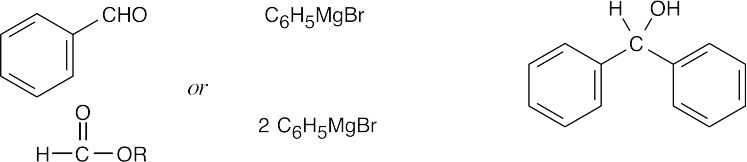 |
|||||
| 10.23 | In general, ketones are less reactive than aldehydes for both steric (excess crowding) and electronic reasons. If the keto aldehyde in this problem were reduced with one equivalent of NaBH4, the aldehyde functional group would be reduced in preference to the ketone.
For the same reason, reaction of the keto aldehyde with one equivalent of ethylene glycol selectively forms the acetal of the aldehyde functional group. The ketone can then be reduced with NaBH4 and the acetal protecting group can be removed.
|
| 10.24 | (a) | 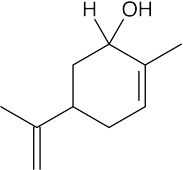 |
(b) | 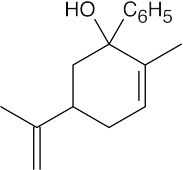 |
(c) | 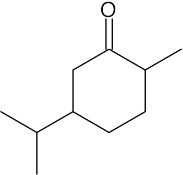 |
| (d) | 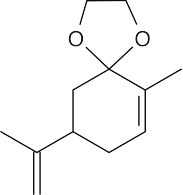 |
General Problems
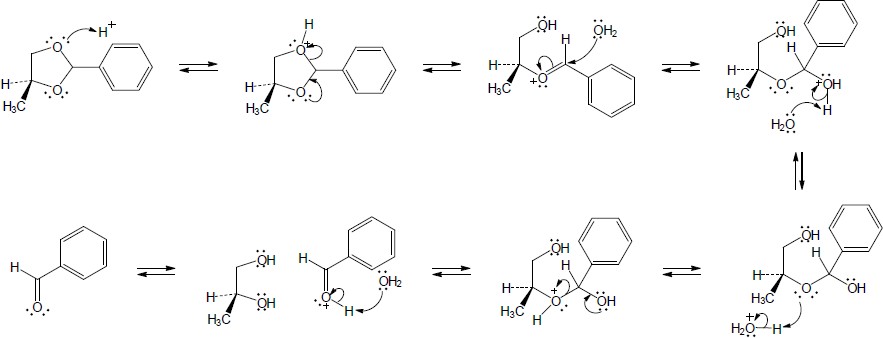
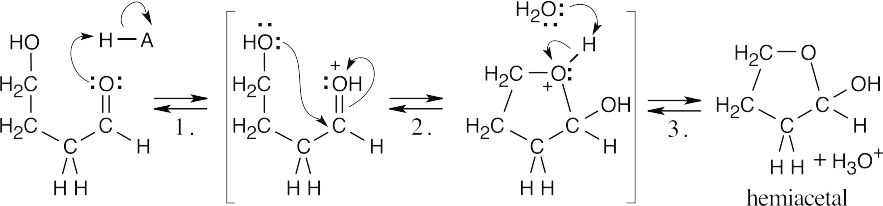
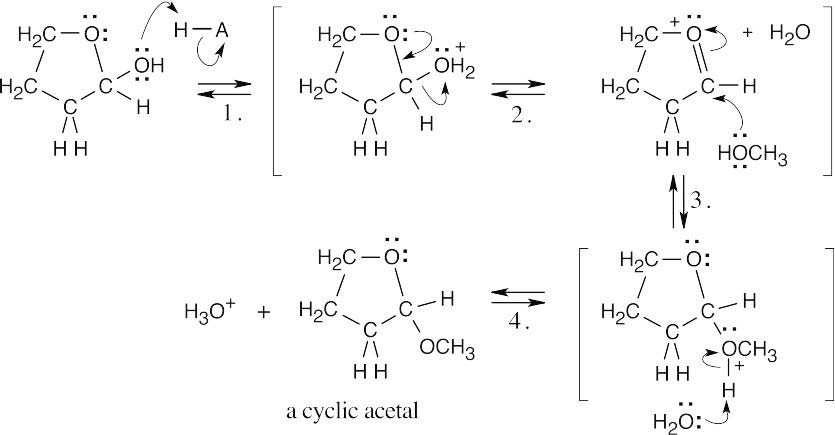
| 10.25 |
4-Hydroxybutanal forms a cyclic hemiacetal when the hydroxyl oxygen adds to the aldehyde group.
|
|
| Step 1: | Protonation. | |
| Step 2: | Addition of –OH. | |
| Step 3: | Loss of proton. | |
|
Methanol reacts with the cyclic hemiacetal to form 2-methoxytetrahydrofuran.
|
||
| Step 1: | Protonation. | |
| Step 2: | Loss of water. | |
| Step 3: | Addition of methanol. | |
| Step 4: |
Loss of proton. | |
| 2-Methoxytetrahydrofuran is a cyclic acetal. The hydroxyl oxygen of 4-hydroxybutanal reacts with the aldehyde to form the cyclic ether linkage. | ||
| 10.26 | 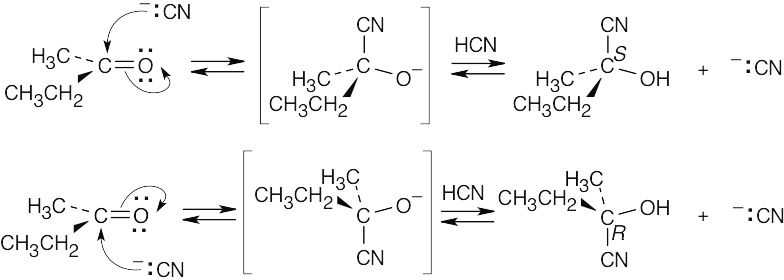
Attack can occur with equal probability on either side of the planar carbonyl group to yield a racemic product mixture that is optically inactive. |