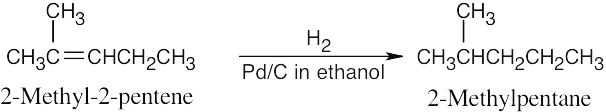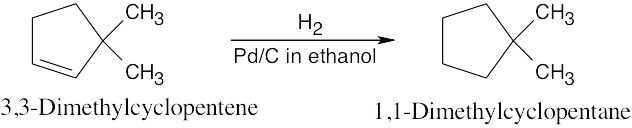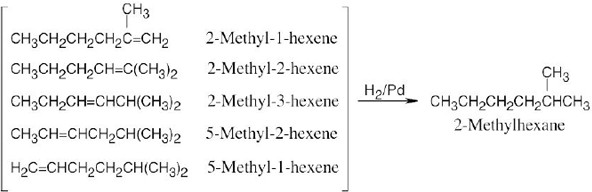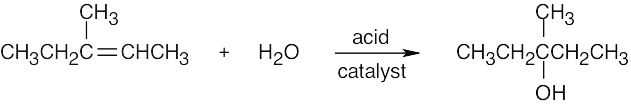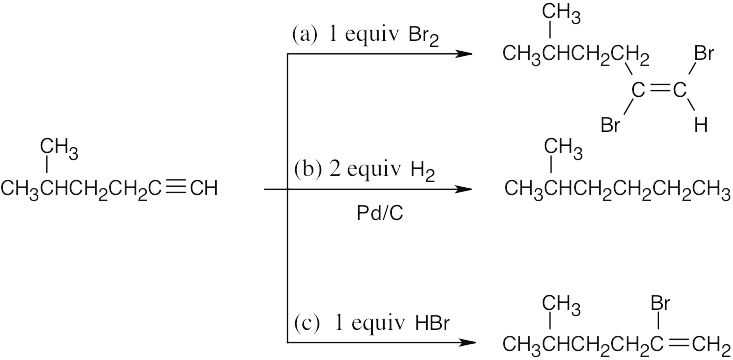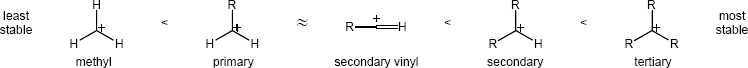5 Chapter 5 Solutions to Problems – Alkenes: Structure and Reactivity
Chapter 5 – Alkenes: Structure and Reactivity
Solutions to Problems
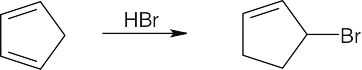
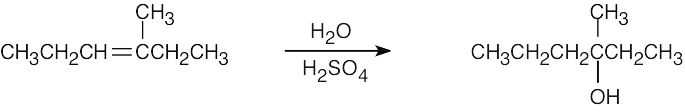
| 5.1 | All of these reactions are electrophilic additions of HX to an alkene. Use Markovnikov’s rule to predict orientation. | |
| (a) |  |
|
| (b) | 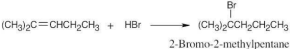
In accordance with Markovnikov’s rule, H forms a bond to the carbon with fewer substituents, and Br forms a bond to the carbon with more substituents. |
|
| (c) | 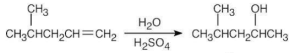 |
|
| (d) | 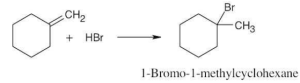 |
|
| 5.2 | Think backward in choosing the alkene starting material for synthesis of the desired haloalkanes. Remember that halogen is bonded to one end of the double bond and that more than one starting material can give rise to the desired product. | |
| (a) | 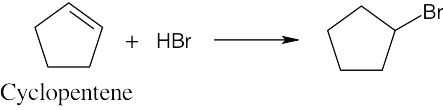 |
|
| (b) | 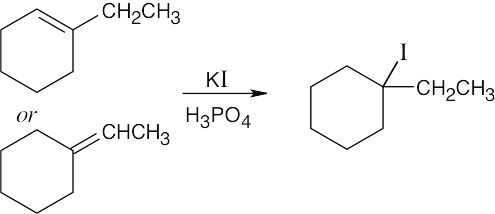 |
|
| (c) | 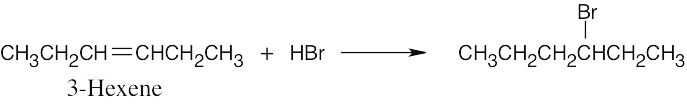 |
|
| (d) | 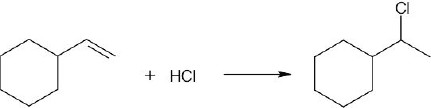 |
|
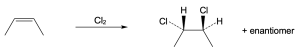
| 5.3 | The more stable carbocation is formed. | |
| (a) | 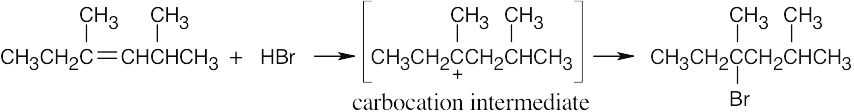 |
|
| (b) | 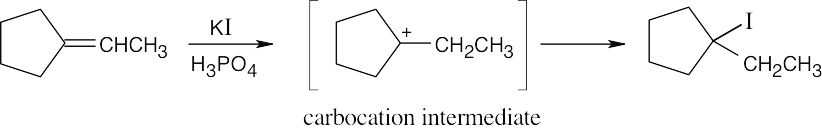 |
|
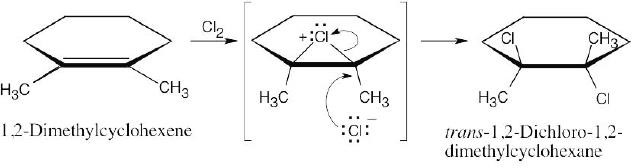
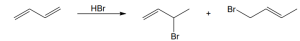
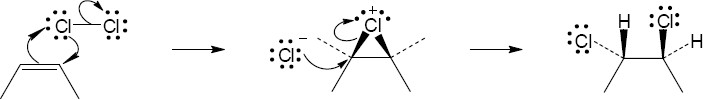
| 5.4 |
The chlorines are trans to one another in the product, as are the methyl groups. |
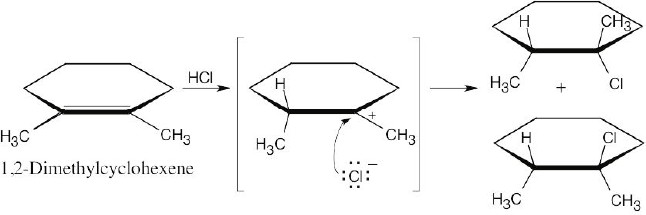
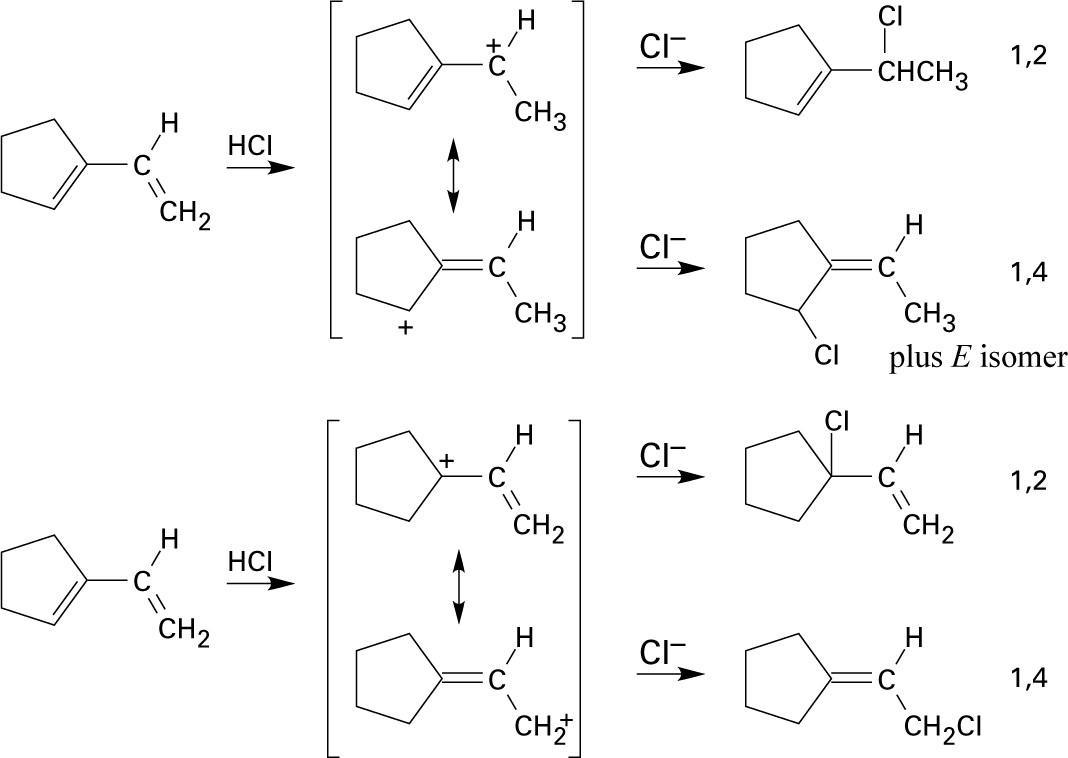
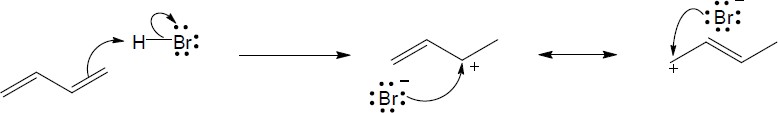
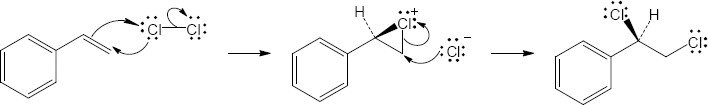
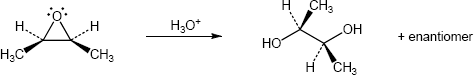
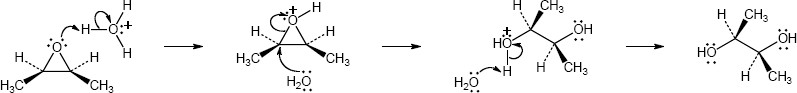
| 5.5 |
Addition of hydrogen halides involves formation of an open carbocation, not a cyclic halonium ion intermediate. The carbocation, which is sp2-hybridized and planar, can be attacked by chloride from either top or bottom, yielding products in which the two methyl groups can be either cis or trans to each other. |
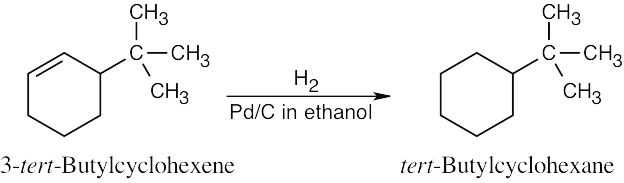
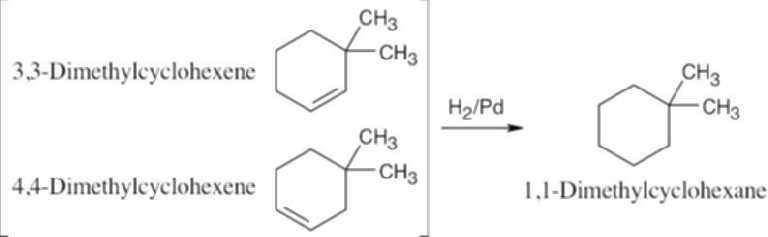
| 5.6 | Catalytic hydrogenation produces alkanes from alkenes.
|
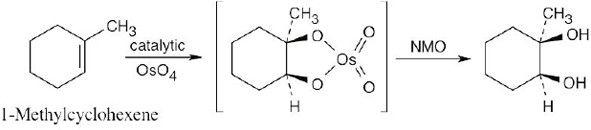
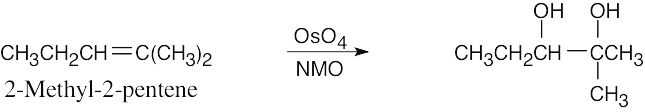
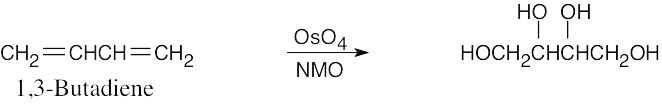
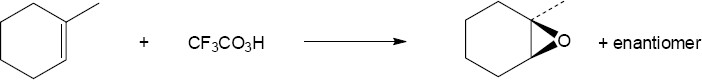
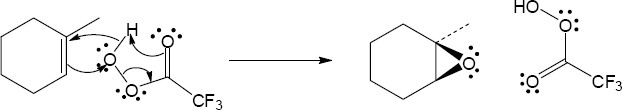
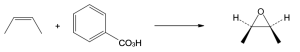
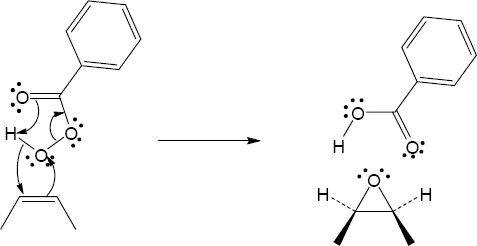
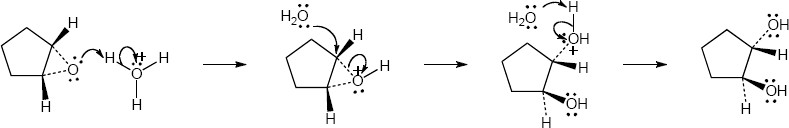
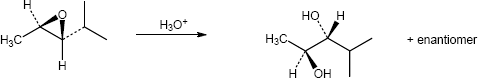
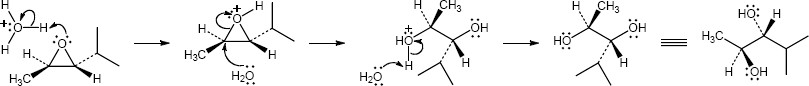
| 5.7 | Reaction of an alkene with a catalytic amount of OsO4, in the presence of N-morpholine N-oxide (NMO), yields a diol product. To pick a starting material for these products, choose an alkene that has a double bond between the diol carbons. The products in (b) and (c) can also be formed by ring opening of an epoxide formed either from a peroxyacid or from a halohydrin.
|
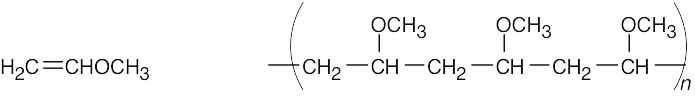
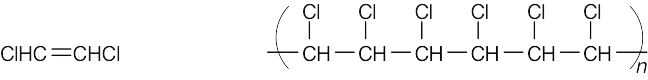
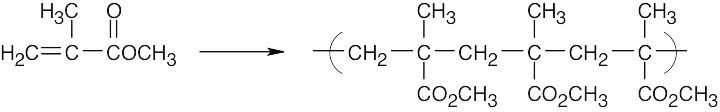
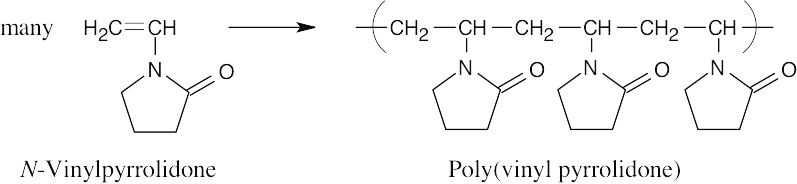
| 5.8 | Find the smallest repeating unit in each polymer and add a double bond. This is the monomer unit.
|
| 5.9 |
|
| 5.10 |
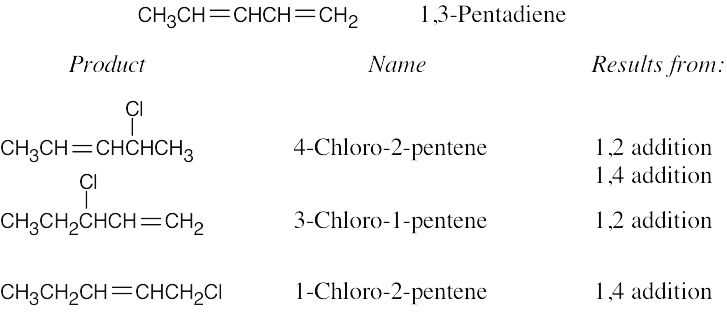
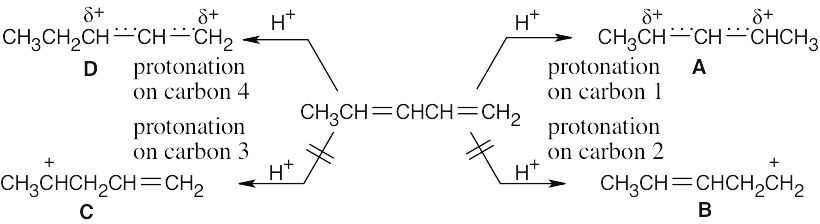
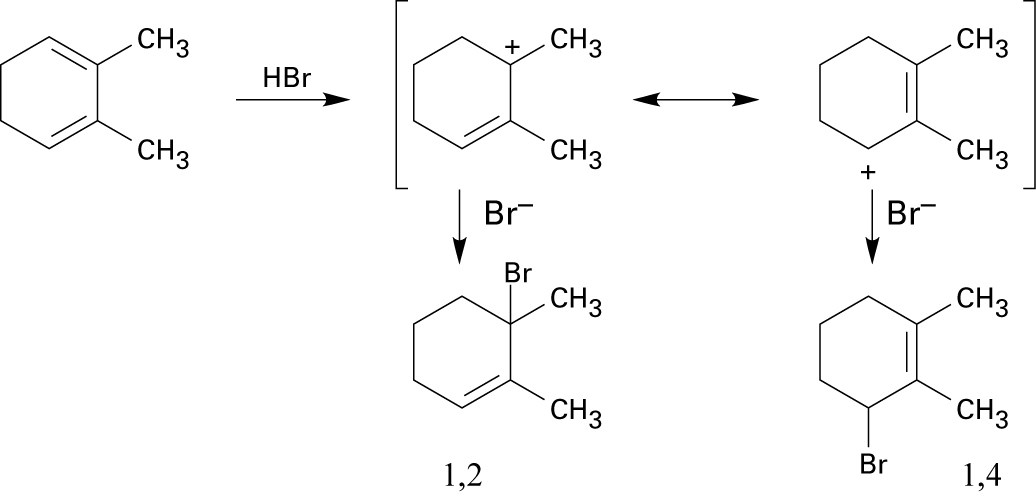
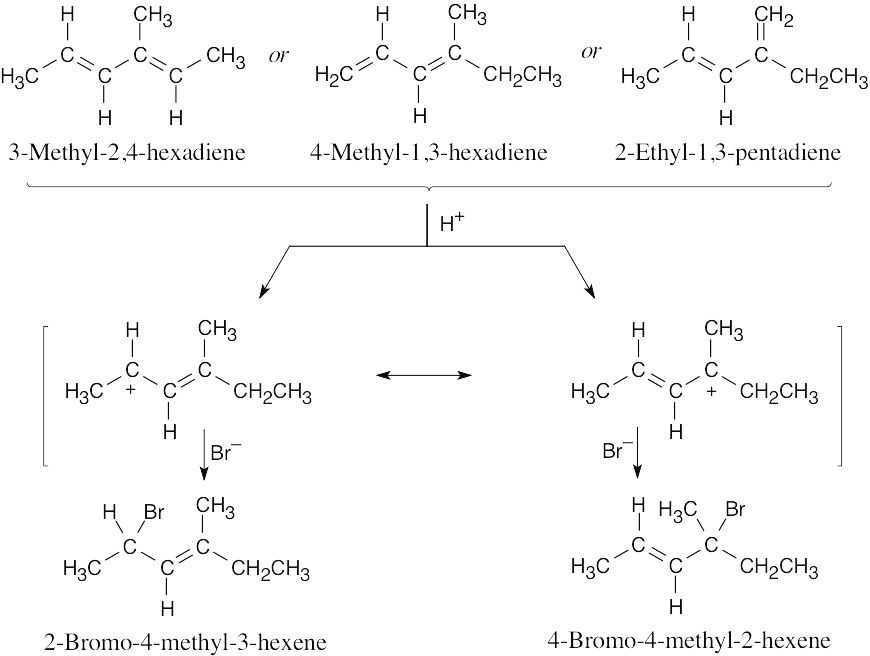
A and D, which are resonance-stabilized, are formed in preference to B and C, which are not. The positive charge of allylic carbocation A is delocalized over two secondary carbons, while the positive charge of carbocation D is delocalized over one secondary and one primary carbon. We therefore predict that carbocation A is the major intermediate formed, and that 4-chloro-2-pentene predominates. Note that this product results from both 1,2 and 1,4 addition. |
| 5.11 |
|
| 5.12 | Markovnikov addition is observed with alkynes as well as with alkenes. | |
| (a) | 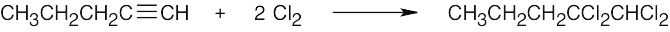 |
|
| (b) | 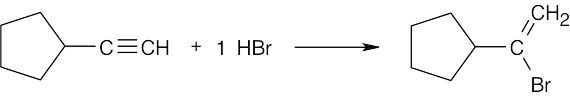 |
|
| (c) | 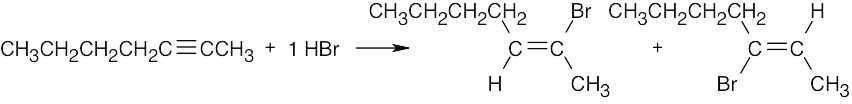
Two products result from addition to an internal alkyne. |
|
| 5.13 | (a) | 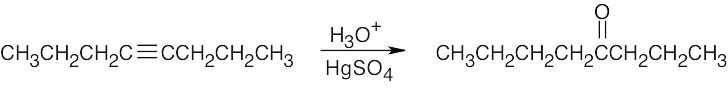
This symmetrical internal alkyne yields only one product. |
| (b) | 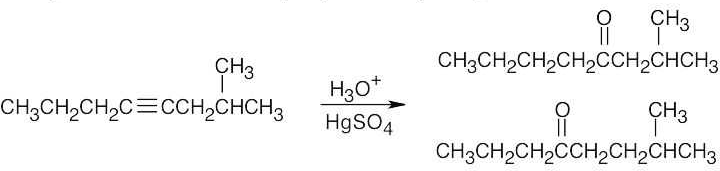
Two ketone products result from hydration of 2-methyl-4-octyne. |
| 5.14 | (a) | 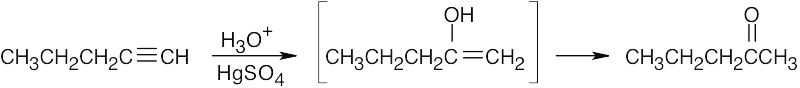 |
| (b) | 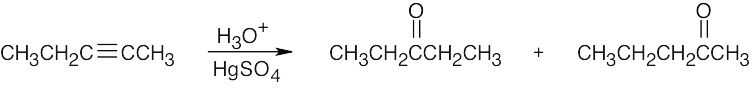
The desired ketone can be prepared only as part of a product mixture. |
| 5.15 | A base that is strong enough to deprotonate acetone must be the conjugate base of an acid weaker than acetone. In this problem, only Na+ –C≡CH (b) is a base strong enough to deprotonate acetone. |
| 5.16 | Remember that the alkyne must be a terminal alkyne and the halide must be primary. More than one combination of terminal alkyne and halide may be possible. | |
| Alkyne R’X (X=Br or I) Product | ||
| (a) | 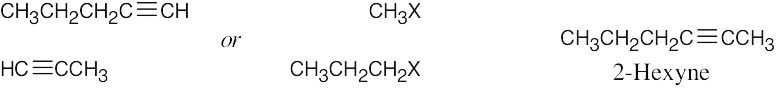 |
|
| (b) | 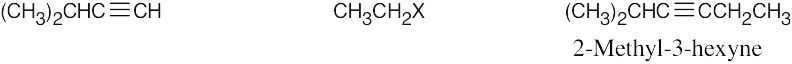 |
|
| (c) | 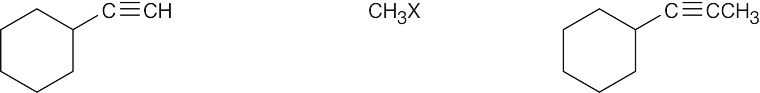
Products (b) and (c) can be synthesized by only one route because only primary halides can be used for acetylide alkylations. |
|
Additional Problems
Visualizing Chemistry
| 5.17 | 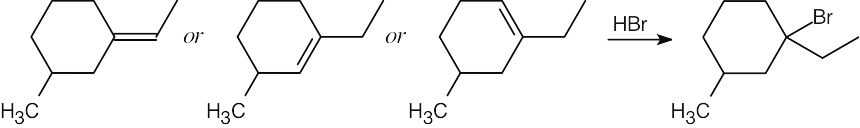 |
| 5.18 | (a) | 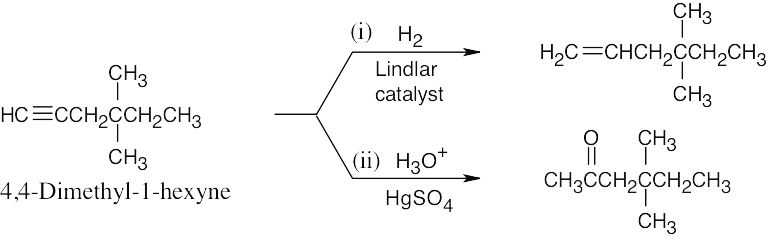 |
| (b) | 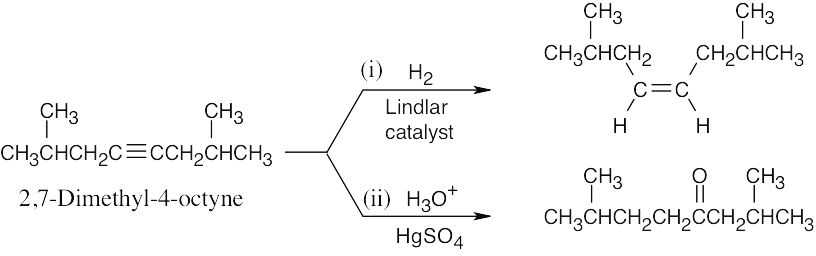 |
| 5.19 | (a) | 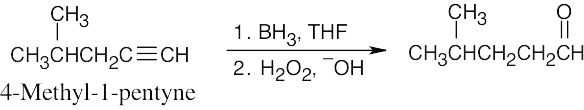
An aldehyde is formed by reacting a terminal alkyne with borane, followed by oxidation. |
| (b) | 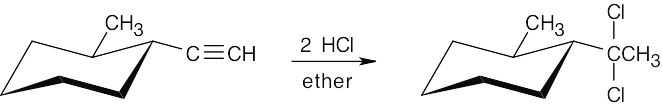 |
| 5.20 | It’s not possible to form a small ring containing a triple bond because the angle strain that would result from bending the bonds of an sp-hybridized carbon to form a small ring is too great. |
Reactions of Alkenes
| 5.21 |
|
| 5.22 |
|
| 5.23 |
|
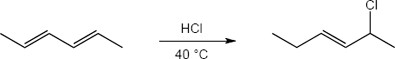
| 5.24 | Note: The major product of the reaction depends on the reaction temperature. At higher temperatures (> 40 °C) the more substituted akene (the thermodynamic product) is favored, while at lower temperatures (0 °C) the less substituted alkene (the kinetic product) is favored.
|
| 5.25 | Any unsubstituted cyclic 1,3-diene cyclic diene gives the same product from 1,2- and 1,4 addition. For example:
|
Mechanism Problems
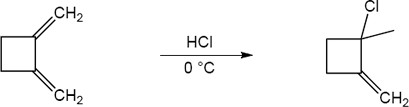
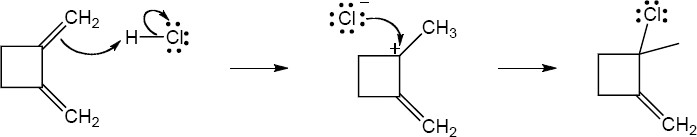
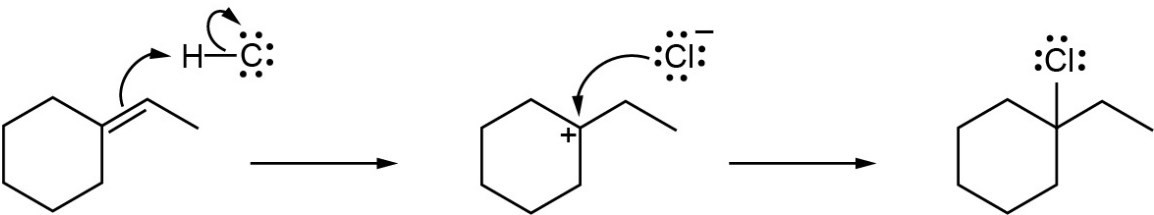
| 5.26 | (a) | 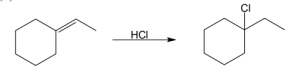
Mechanism:
|
| (b) | 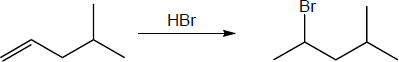
Mechanism:
|
|
| (c) | 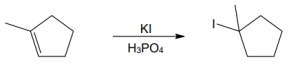
Mechanism:
|
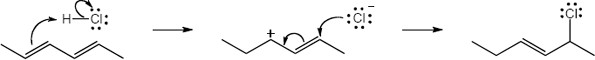
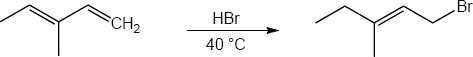
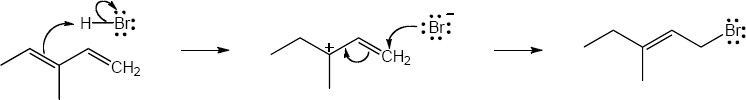
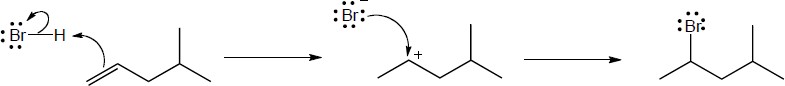
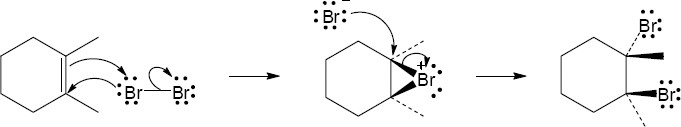
| 5.27 |
Note: Because the carbocation intermediate is resonance stabilized, there are two locations where the bromine can add.
Mechanism:
|
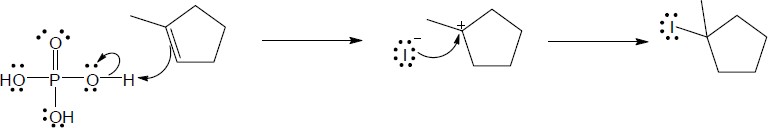
| 5.28 |
|
| 5.29 |
|
| 5.30 |
|
Carbocations and Electrophilic Addition Reactions
| 5.31 | The order of carbocation stability is below.
|
|
| (a) | 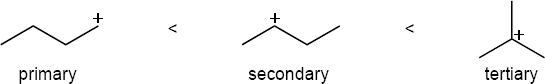 |
|
| (b) | 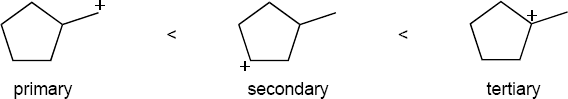 |
|
| (c) | 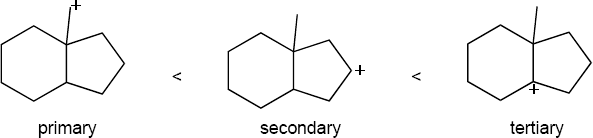 |
|
| 5.32 | (a) |
|
| (b) |  |
| 5.33 | (a) |
|
| (b) | 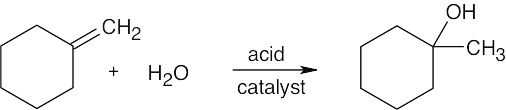 |
|
| (c) | 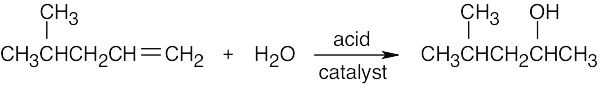 |
Polymers
| 5.34 |
|
| 5.35 |
|
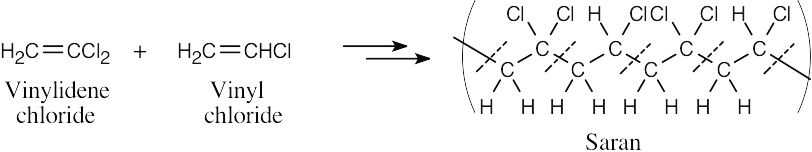
| 5.36 |
The dashed lines cross the bonds formed during the polymerization reaction. The structural fragments that lie between the dashed lines are the monomer units. Saran is a copolymer of vinylidene chloride and vinyl chloride. |
Reactions of Alkynes
| 5.37 |
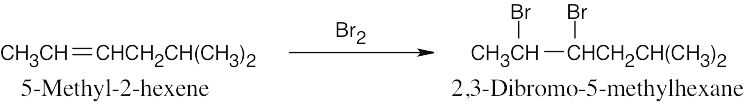
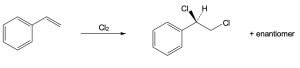
Addition of one equivalent of HX or X2 to a triple bond occurs with Markovnikov regiochemistry to yield a product in which the two added atoms usually have a trans relationship across the double bond. |
| 5.38 | 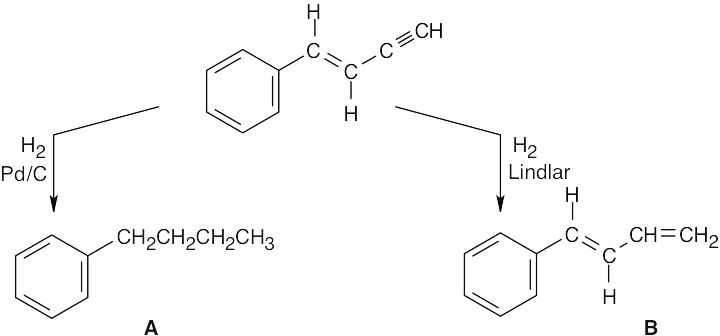 |
| 5.39 | (a) | 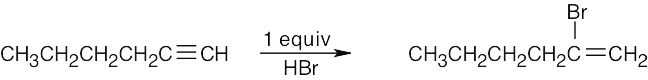 |
| (b) | 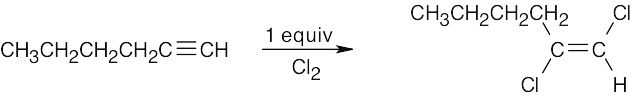 |
|
| (c) | 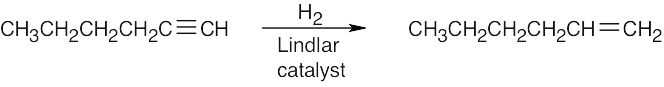 |
|
| (d) | 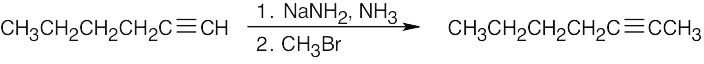 |
|
| (e) |  |
|
| (f) | 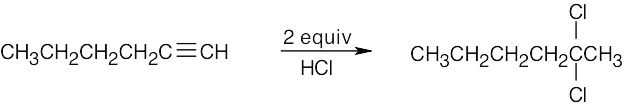 |
| 5.40 | (a) | 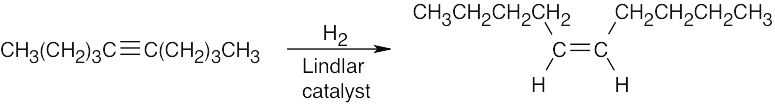 |
| (b) | 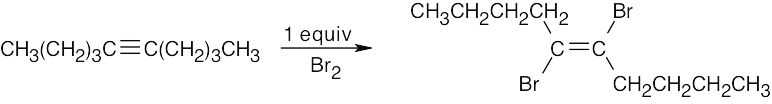 |
|
| (c) | 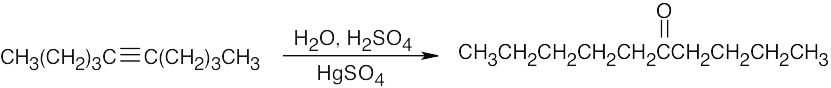 |
|
| (d) | 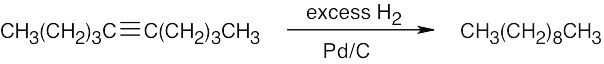 |
| 5.41 | Mixtures of products are sometimes formed since the alkynes are unsymmetrical. | |
| (a) | 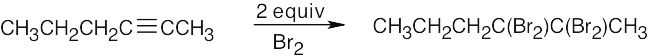 |
|
| (b) | 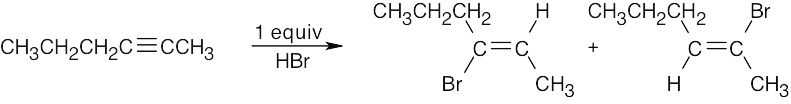 |
|
| (c) | 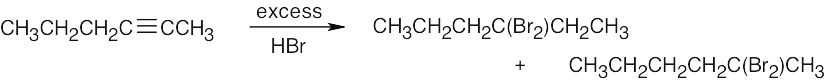 |
|
| (d) | 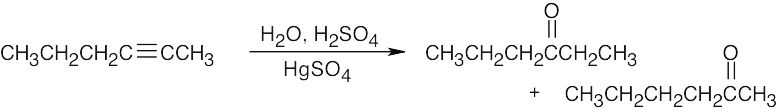 |
|
| 5.42 | (a) | 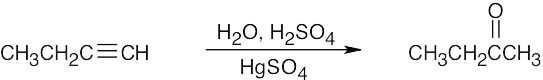 |
| (b) | 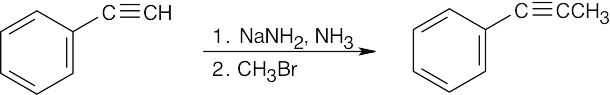 |
|
| (c) | 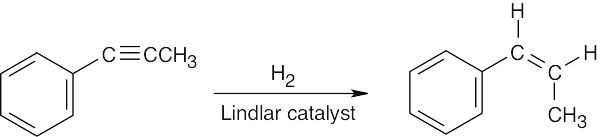 |
| 5.43 |
The overall trend for carbocation stability is shown below. Recall that resonance adds additional stability to carbocations.
|
|
| (a) | 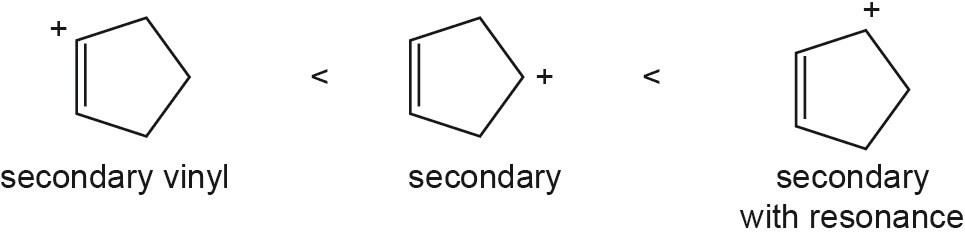 |
|
| (b) | 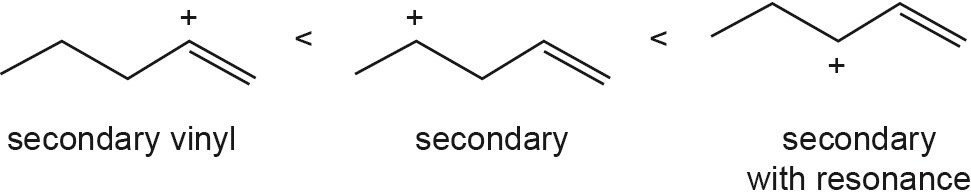 |
|
| (c) | 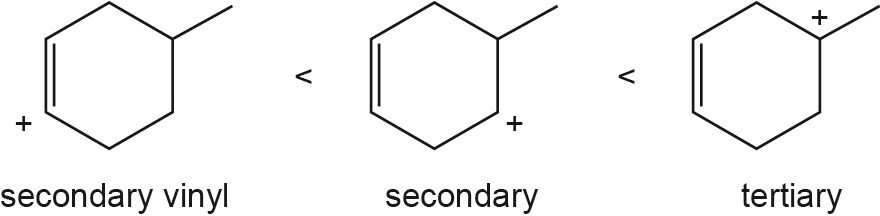 |
|