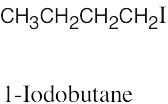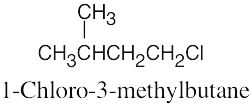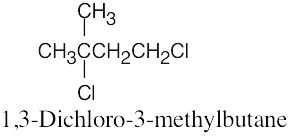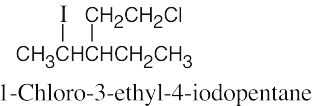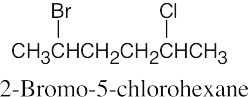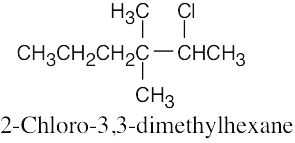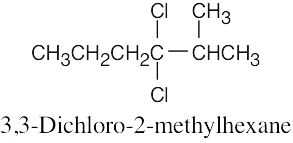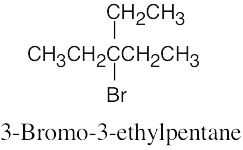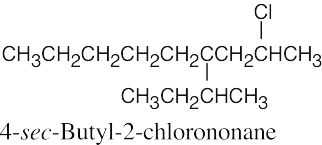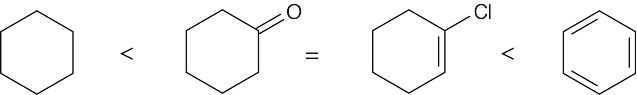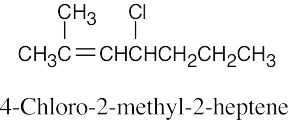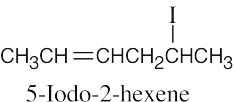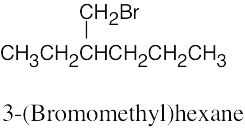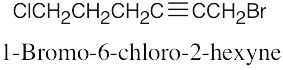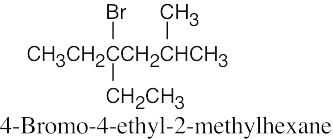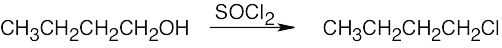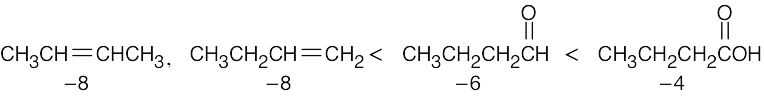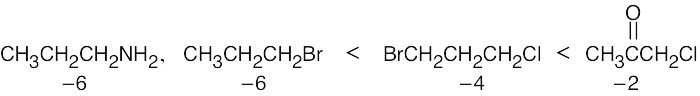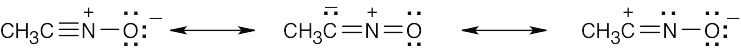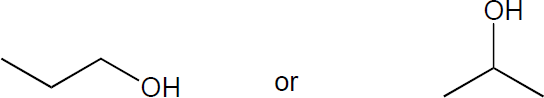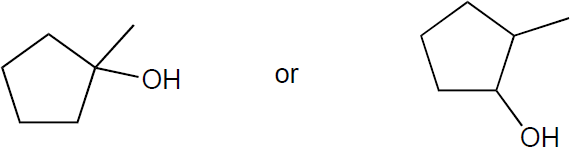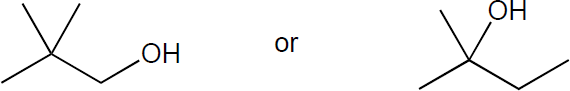6 Chapter 6 Solutions to Problems – Organohalides
Chapter 6 – Organohalides
Solutions to Problems
| 6.1 | The rules that were given for naming alkanes in Section 1.15 are used for alkyl halides. A halogen is treated the same as an alkyl substituent but is named as a halo group.
|
| 6.2 |
|
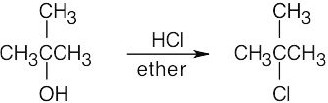
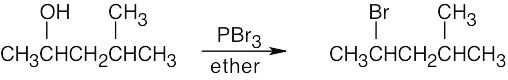
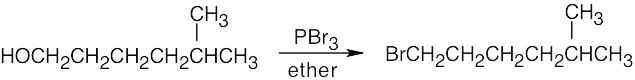
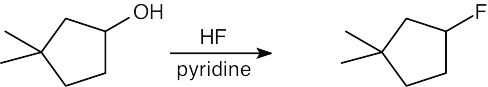
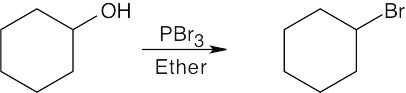
| 6.3 | Remember that halogen acids are used for converting tertiary alcohols to alkyl halides. PBr3 and SOCl2 are used for converting secondary and primary alcohols to alkyl halides. (CH3CH2)2NSF3 and HF in pyridine can be used to form alkyl fluorides.
|
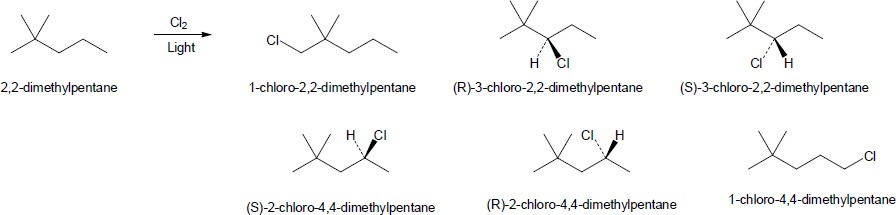
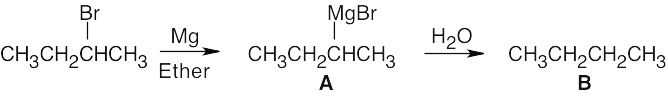
| 6.4 | Table 5.2 shows that the pKa of CH3–H is 60. Since CH4 is a very weak acid, –:CH3 is a very strong base. Alkyl Grignard reagents are similar in base strength to –:CH3, but alkynyl Grignard reagents are somewhat weaker bases. Both reactions (a) and (b) occur as written.
|
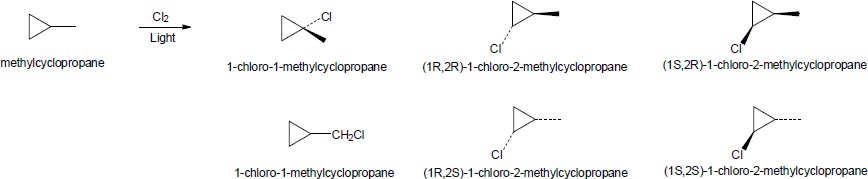
| 6.5 |
|
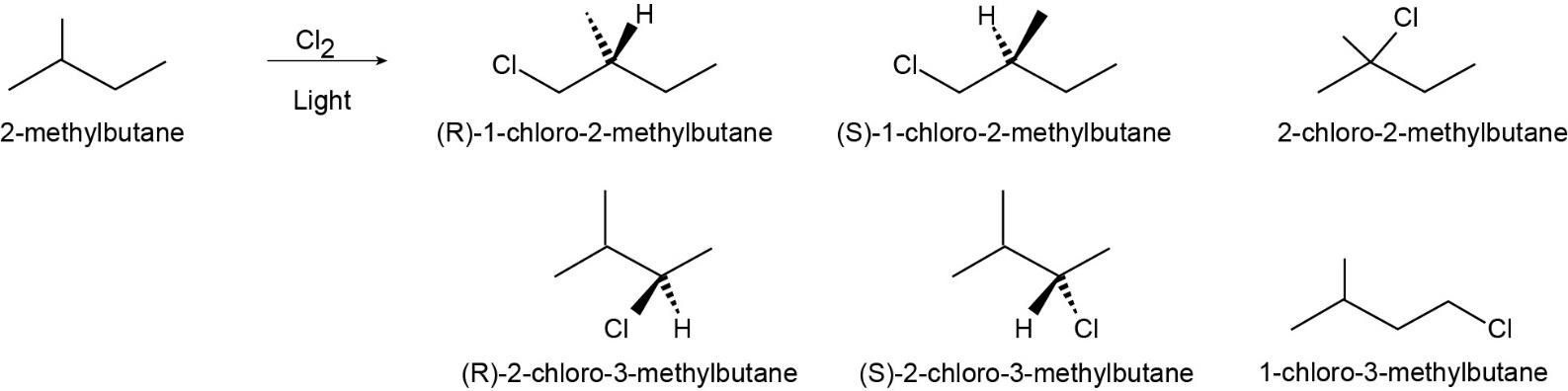
| 6.6 |
|
Additional Problems
Visualizing Chemistry
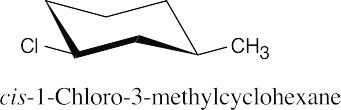
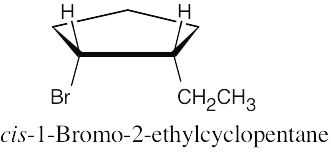
| 6.7 |
|
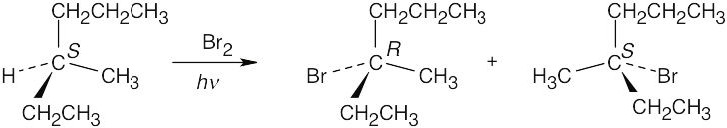
| 6.8 | The name of the compound is (R)-2-bromopentane. Reaction of (S)-2-pentanol with PBr3 to form (R)-2-bromopentane occurs with a change in stereochemistry because the configuration at the chirality center changes from S to R. |
Mechanism Problems
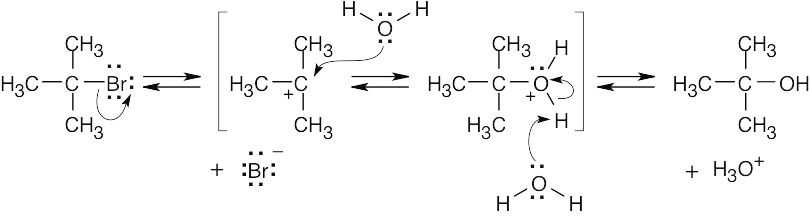
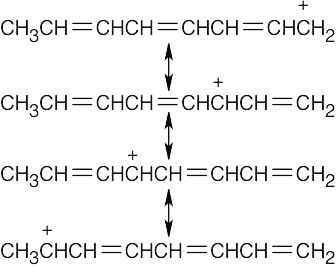
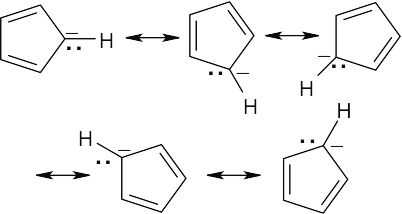
| 6.9 |
|
Naming Alkyl Halides
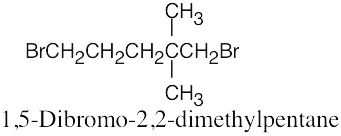
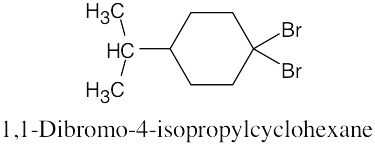
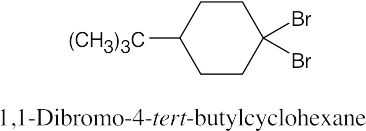
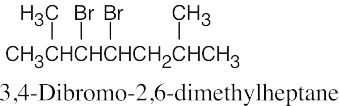
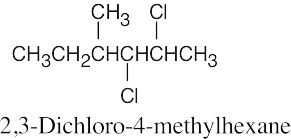
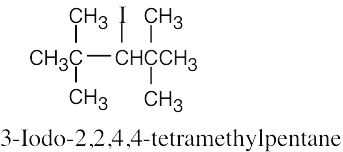
| 6.10 |
|
| 6.11 |
|
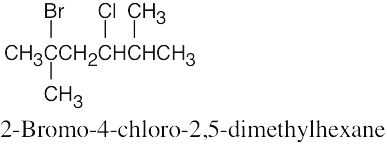
| 6.12 |
|
Synthesizing Alkyl Halides
| 6.13 |
|
| 6.14 |
|
Oxidation and Reduction
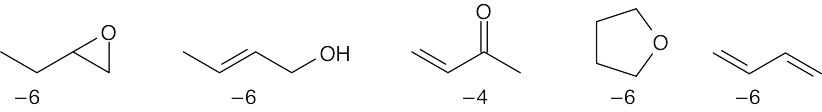
| 6.15 | Remember that the oxidation level is found by subtracting the number of C–H bonds from the number of C–O, C–N, and C–X bonds. The oxidation levels are shown beneath the structures.
In order of increasing oxidation level:
|
| 6.16 |
All of the compounds except 3 (which is more oxidized) have the same oxidation level. |
| 6.17 |
|
General Problems
| 6.18 |
|
| 6.19 |
Abstraction of a hydrogen atom from the chirality center of (S)-3-methylhexane produces an achiral radical intermediate, which reacts with bromine to form a 1:1 mixture of R and S enantiomeric, chiral bromoalkanes. The product mixture is optically inactive. |
| 6.20 |
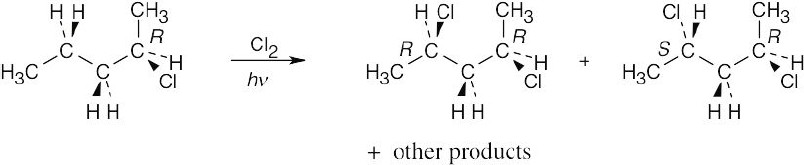
Abstraction of a hydrogen atom from carbon 4 yields a chiral radical intermediate. Reaction of this intermediate with chlorine does not occur with equal probability from each side, and the two diastereomeric products are not formed in a 1:1 ratio. The first product is optically active, and the second product is a meso compound. |
| 6.21 |
|
| 6.22 | A Grignard reagent cannot be prepared from a compound containing an acidic functional group because the Grignard reagent is immediately quenched by the proton source. For example, the –CO2H, –OH, –NH2, and RC≡CH functional groups are too acidic to be used for preparation of a Grignard reagent. BrCH2CH2CH2NH2 is another compound that does not form a Grignard reagent. |
| 6.23 |
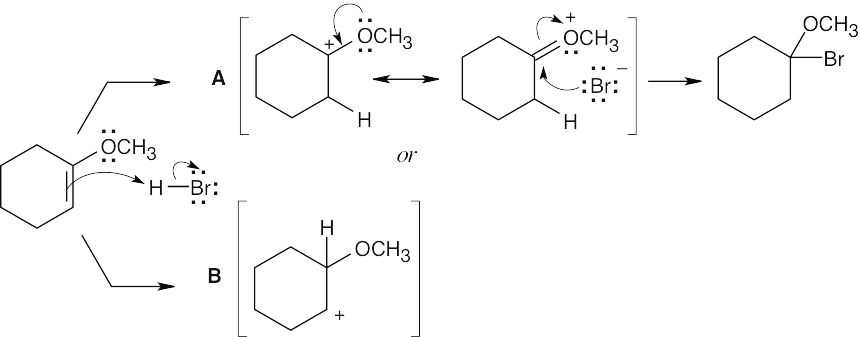
Reaction of the ether with HBr can occur by either path A or path B. Path A is favored because its cation intermediate can be stabilized by resonance. |
| 6.24 | Tertiary carbocations (R3C+) are more stable than either secondary or primary carbocations, due to the ability of the three alkyl groups to stabilize a positive charge. If the substrate is also allylic, as in the case of H2C=CHC(CH3)2Br, positive charge can be further delocalized. Thus, H2C=CHC(CH3)2Br should form a carbocation faster than (CH3)3CBr because the resulting carbocation is more stable. |
| 6.25 |
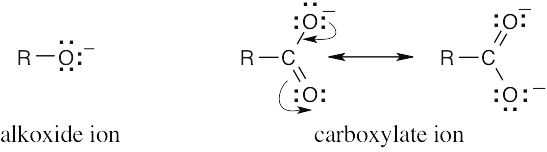
Carboxylic acids are more acidic than alcohols because the negative charge of a carboxylate ion is stabilized by resonance. This resonance stabilization favors formation of carboxylate anions over alkoxide anions, and increases Ka for carboxylic acids. Alkoxide anions are not resonance stabilized. |
| 6.26 |
|