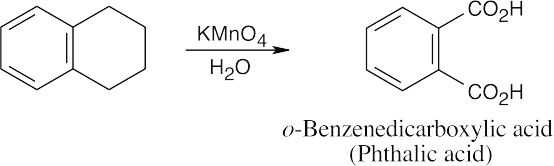
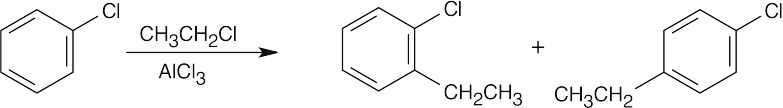
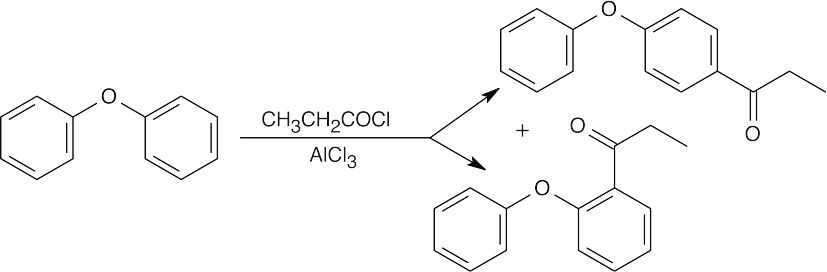
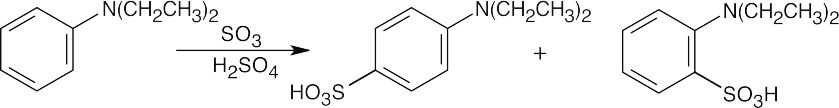
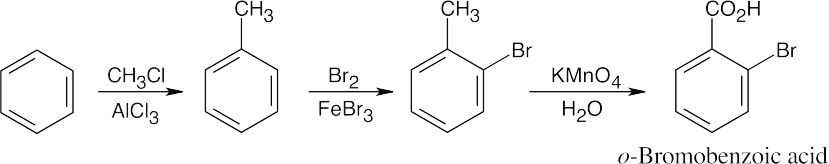
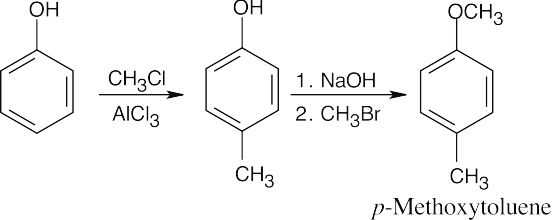
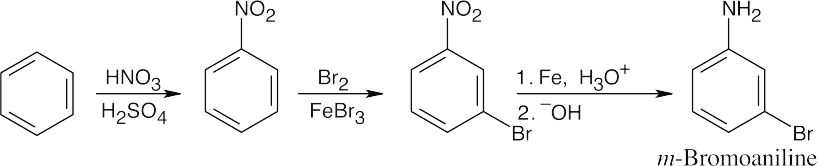
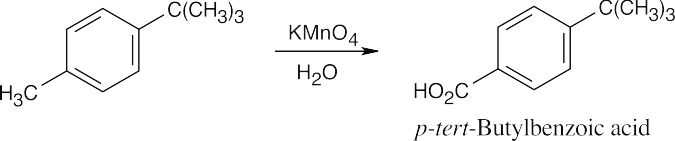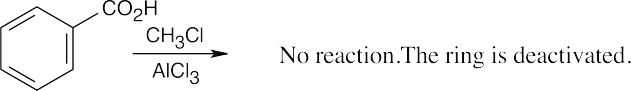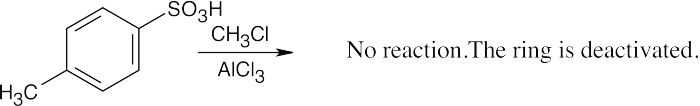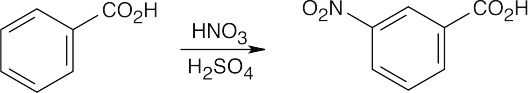8 Chapter 8 Solutions to Problems – Benzene and Aromaticity
Chapter 8 – Benzene and Aromaticity
Solutions to Problems
| 8.1 | 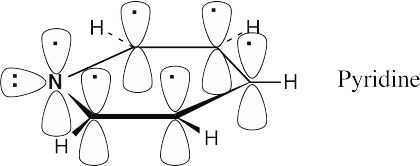
The electronic descriptions of pyridine and benzene are very similar. The pyridine ring is formed by the σ overlap of carbon and nitrogen sp2 orbitals. In addition, six p orbitals, perpendicular to the plane of the ring, hold six electrons. These six p orbitals form six π molecular orbitals that allow electrons to be delocalized over the π system of the pyridine ring. The lone pair of nitrogen electrons occupies an sp2 orbital that lies in the plane of the ring. |
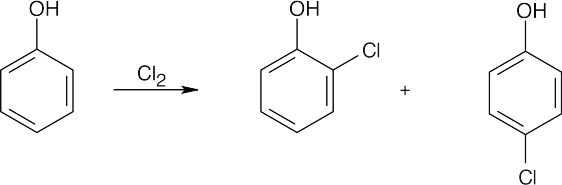
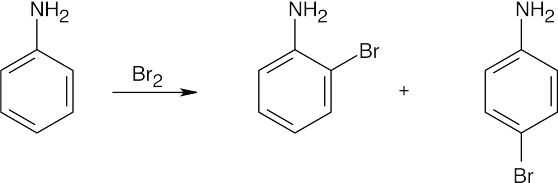
| 8.2 | An ortho disubstituted benzene has two substituents in a 1,2 relationship. A meta disubstituted benzene has two substituents in a 1,3 relationship. A para disubstituted benzene has two substituents in a 1,4 relationship. | |||||
| (a) | 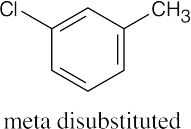 |
(b) | 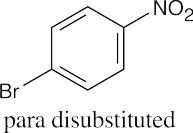 |
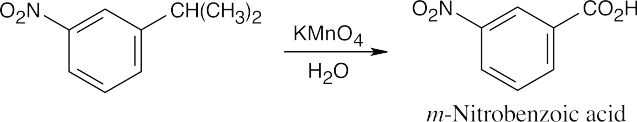
(c) | 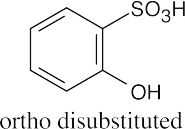 |
|
| 8.3 | Remember to give the lowest possible numbers to substituents on trisubstituted rings. | |||||
| (a) | 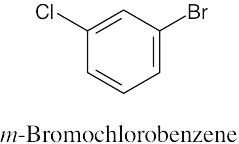 |
(b) | 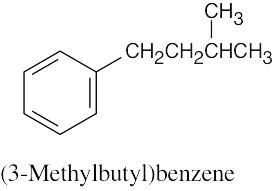 |
(c) | 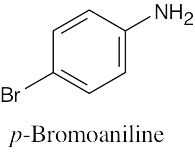 |
|
| (d) | 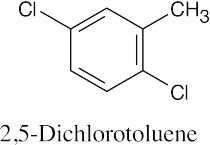 |
(e) | 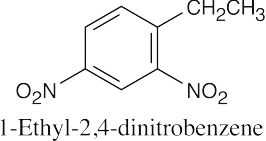 |
(f) | 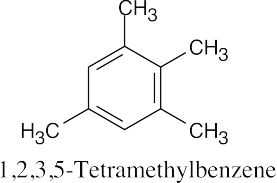 |
|
| 8.4 | (a) | 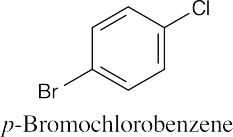 |
(b) | 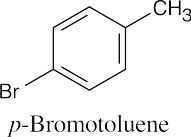 |
(c) | 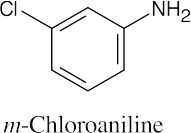 |
| (d) | 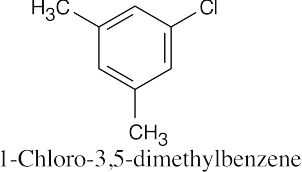 |
| 8.5 |
|
| 8.6 |
|
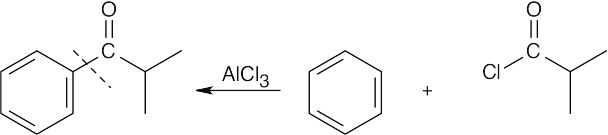
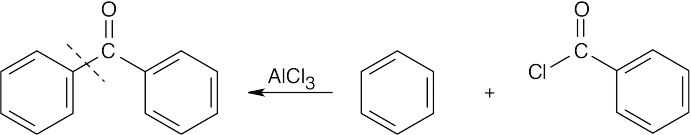
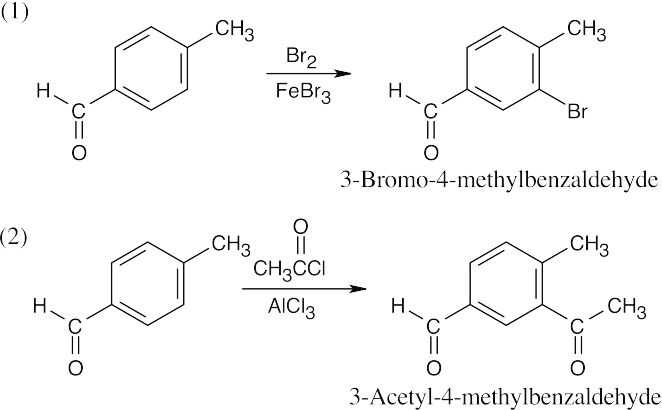
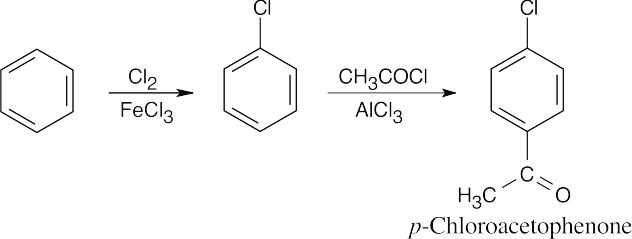
| 8.7 | To identify the carboxylic acid chloride used in the Friedel–Crafts acylation of benzene, break the bond between benzene and the ketone carbon and replace it with a –Cl.
|
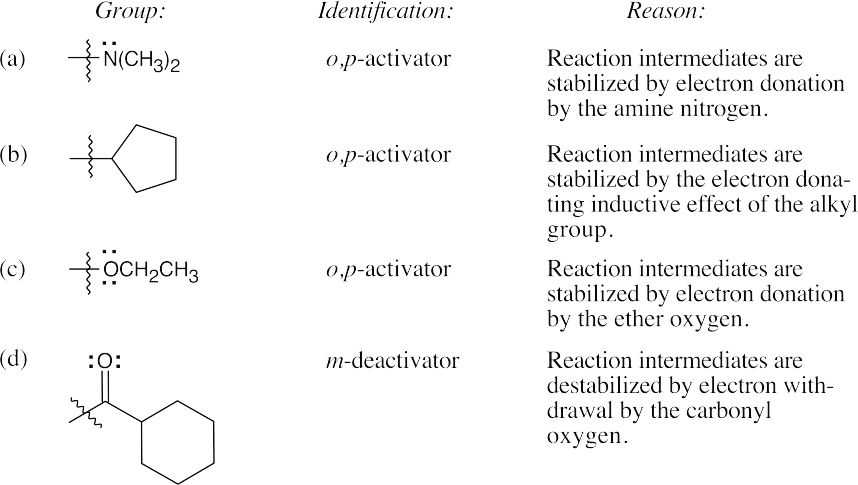
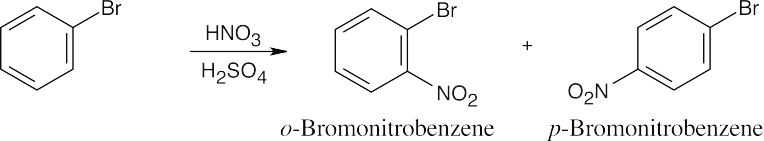
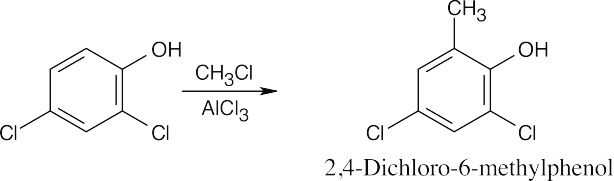
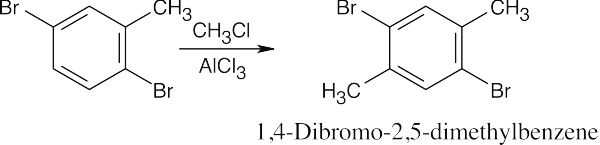
| 8.8 | Use Figure 8.11 to find the activating and deactivating effects of groups.
Most Reactive ———————> Least Reactive
|
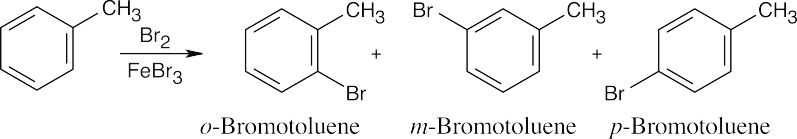
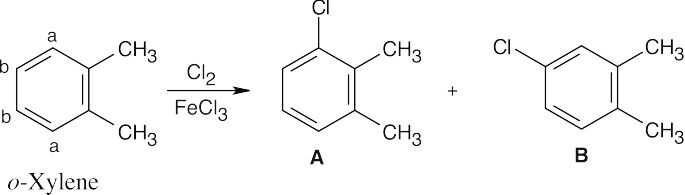
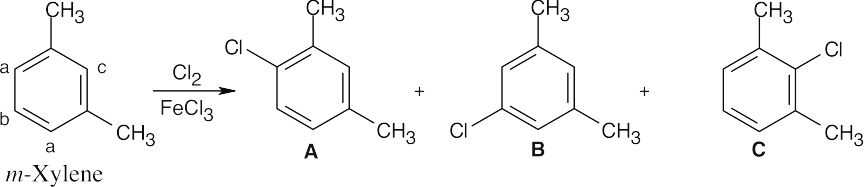
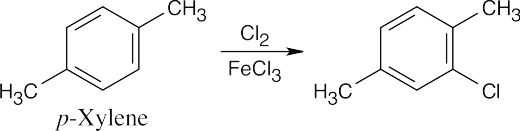
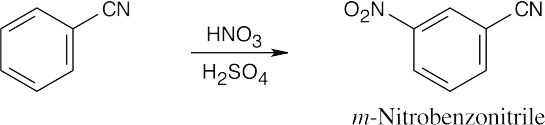
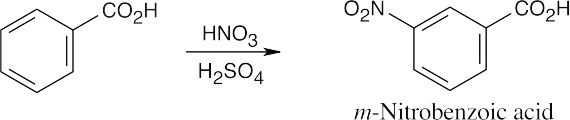
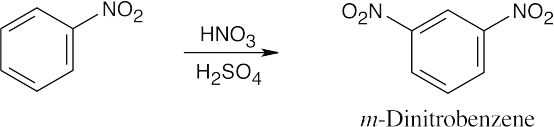
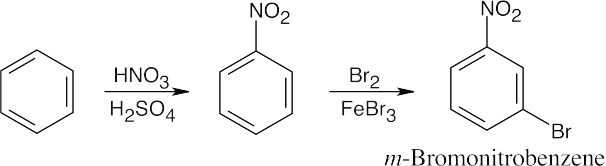
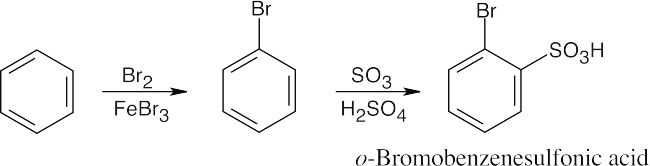
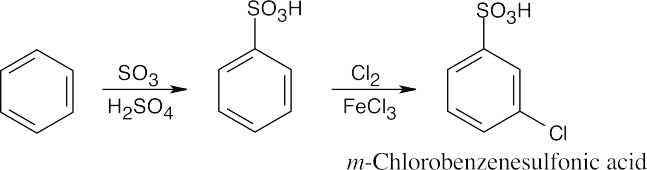
| 8.9 | Refer to Figure 8.11 in the text for the directing effects of substituents. You should memorize the effects of the most important groups. As in Worked Example 8.1, identify the directing effect of the substituent, and draw the product.
|
| 8.10 | (Trifluoromethyl) benzene is less reactive toward electrophilic substitution than toluene. The electronegativity of the three fluorine atoms causes the trifluoromethyl group to be electron-withdrawing and deactivating toward electrophilic substitution. The electrostatic potential map shows that the aromatic ring of (trifluoromethyl) benzene is more electron-poor, and thus less reactive, than the ring of toluene (red). |
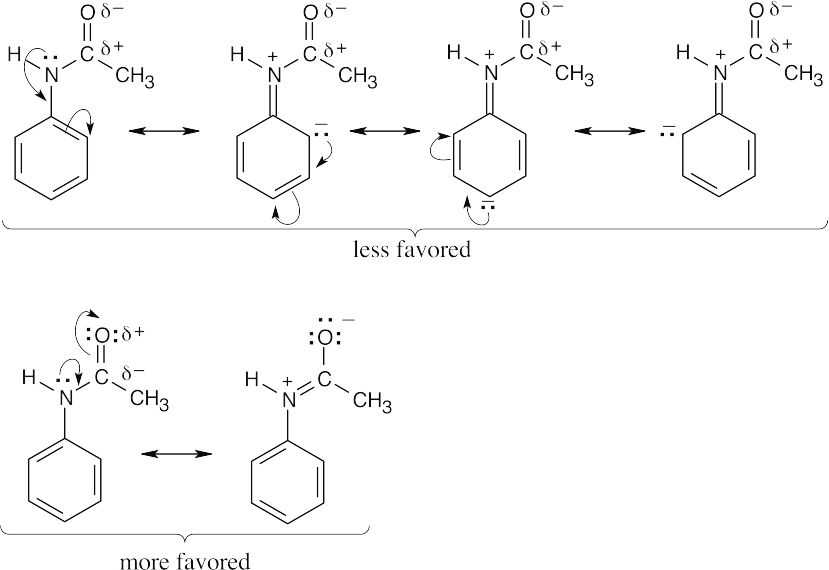
| 8.11 |
For acetanilide, resonance delocalization of the nitrogen lone pair electrons to the aromatic ring is less favored because the positive charge on nitrogen is next to the positively polarized carbonyl group. Resonance delocalization to the carbonyl oxygen is favored because of the electronegativity of oxygen. Since the nitrogen lone pair electrons are less available to the ring than in aniline, the reactivity of the ring toward electrophilic substitution is decreased, and acetanilide is less reactive than aniline toward electrophilic substitution. |
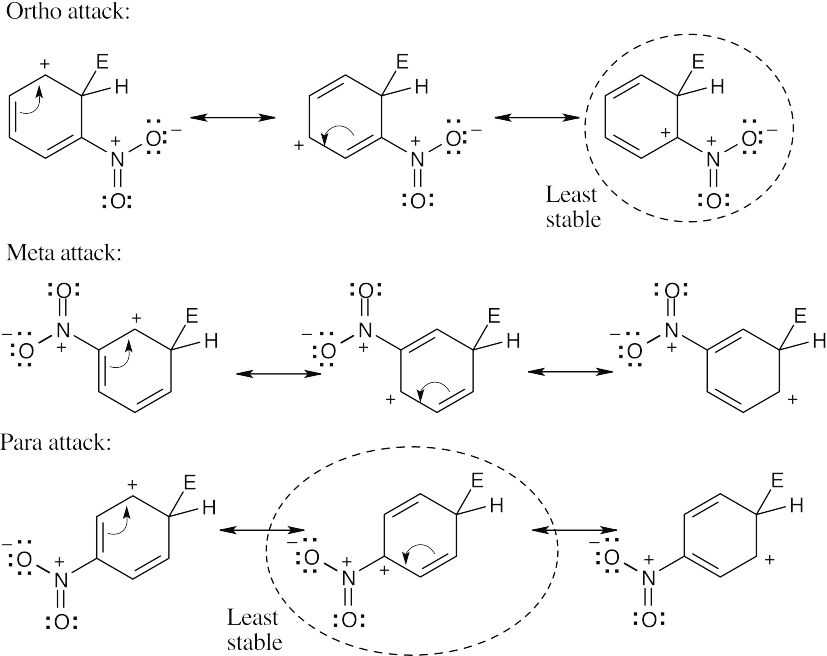
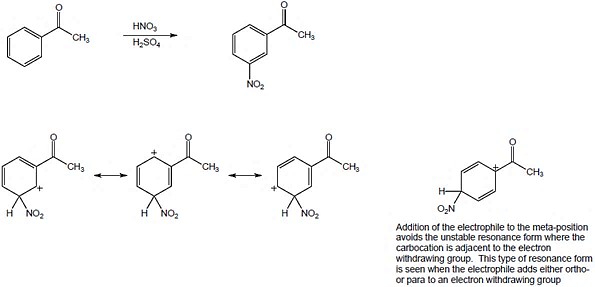
| 8.12 |
The circled resonance forms are unfavorable, because they place two positive charges adjacent to each other. The intermediate from meta attack is thus favored. |
| 8.13 | Oxidation takes place at the benzylic position.
Treatment with KMnO4 oxidizes the methyl group but leaves the tert-butyl group untouched. |
Additional Problems
Visualizing Chemistry
| 8.14 | (a) | 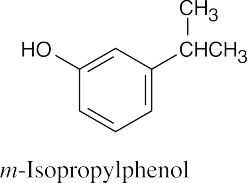 |
(b) | 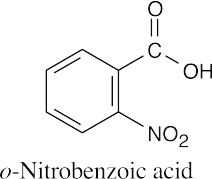 |
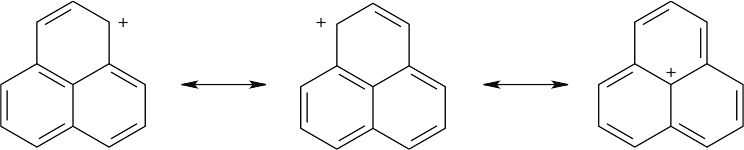
| 8.15 | Three resonance forms for the carbocation of the formula C13H9 are shown below, and more can be drawn. These forms show that the positive charge of the carbocation can be stabilized in the same way as an allylic or benzylic carbocation is stabilized – by overlap with the neighboring π electrons of the ring system.
|
| 8.16 | 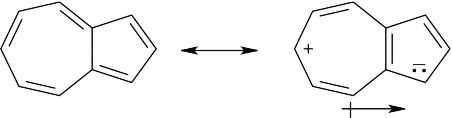
Molecules with dipole moments are polar because electron density is drawn from one part of the molecule to another. In azulene, for example, electron density is drawn from the seven-membered ring to the five-membered ring, satisfying Hückel’s rule for both rings and producing a dipole moment. The five-membered ring resembles the cyclopentadienyl anion in having six π electrons, while the seven-membered ring resembles the cycloheptatrienyl cation. The electrostatic potential map shows that the five-membered ring is more electron-rich (red) than the seven-membered ring. |
| 8.17 |
|
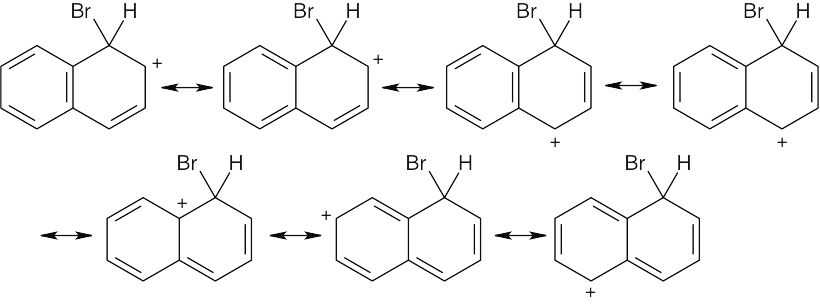
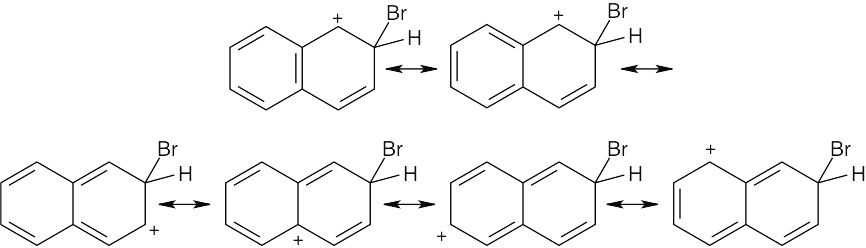
| 8.18 |
|
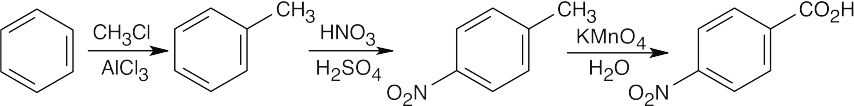
| 8.19 | Imagine two routes for synthesis of m-nitrotoluene:
Thus, it isn’t possible to synthesize m-nitrotoluene by any route that we have studied in this chapter. |
Naming Aromatic Compounds
| 8.20 | (a) | 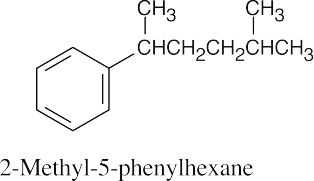 |
(b) | 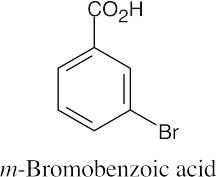 |
(c) | 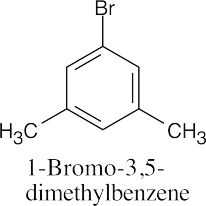 |
| (d) | 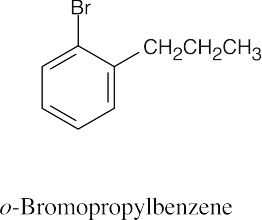 |
(e) | 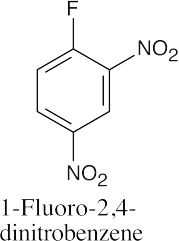 |
(f) | 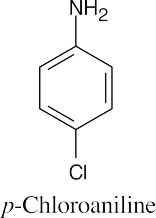 |
| 8.21 | (a) | 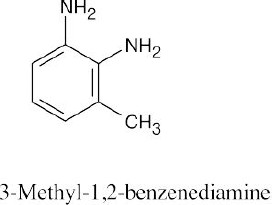 |
(b) | 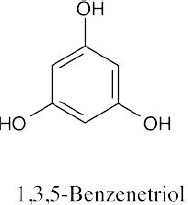 |
(c) | 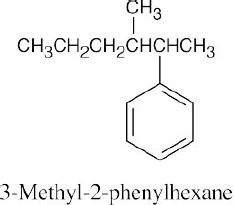 |
| (d) | 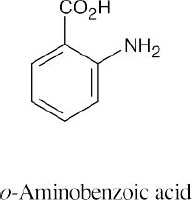 |
(e) | 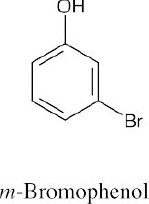 |
(f) | 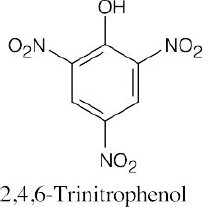 |
| 8.22 | (a) | 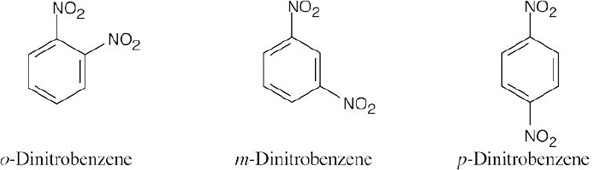 |
| (b) | 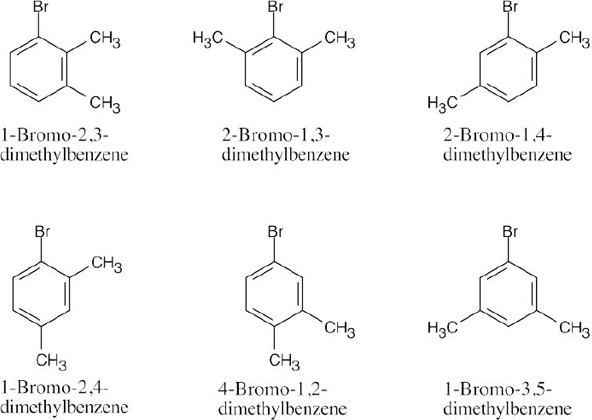 |
|
| (c) | 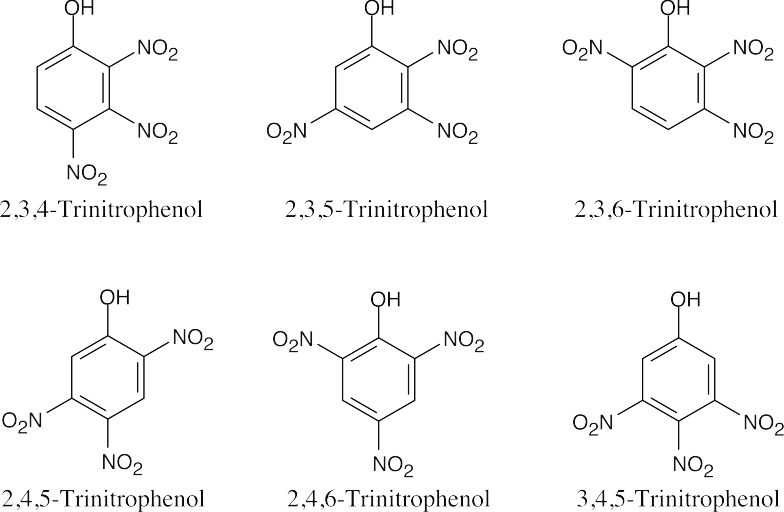 |
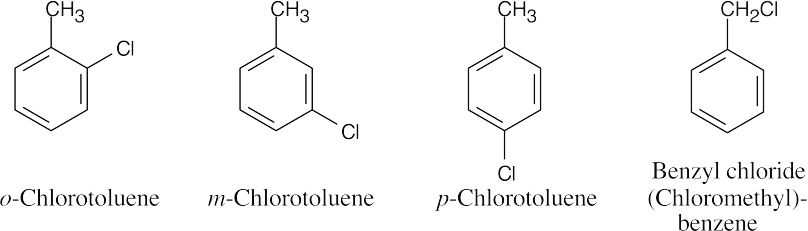
| 8.23 | All aromatic compounds of formula C7H7Cl have one ring and three double bonds.
|
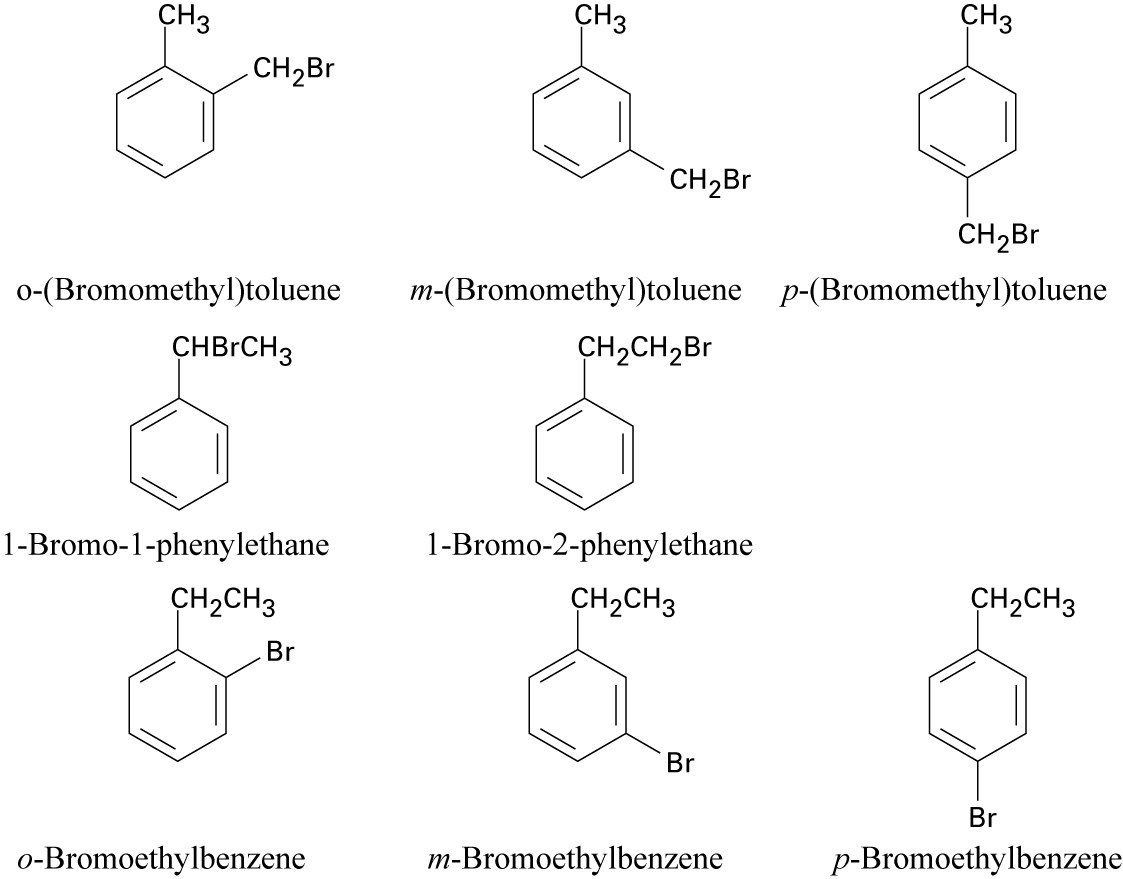
| 8.24 |
Six of these compounds are illustrated and named in Problem 8.22 (b).The other eight are:
|
Structures of Organic Compounds
| 8.25 | All compounds in this problem have four double bonds and/or rings and must be substituted benzenes, if they are to be aromatic. They may be substituted by methyl, ethyl, propyl, or butyl groups. | |
| (a) | 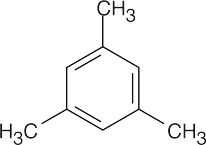 |
|
| (b) | 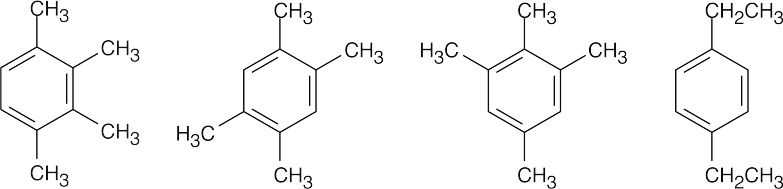 |
|
| (c) | 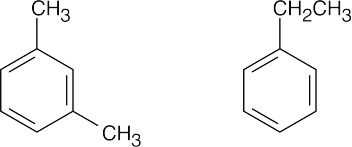 |
|
| (d) | 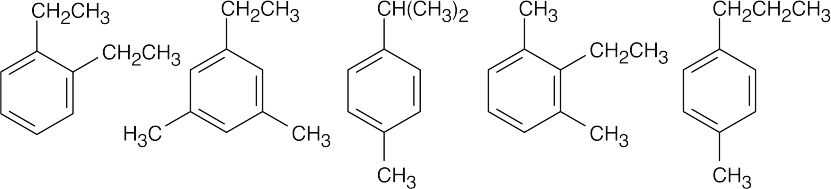 |
|
| 8.26 | 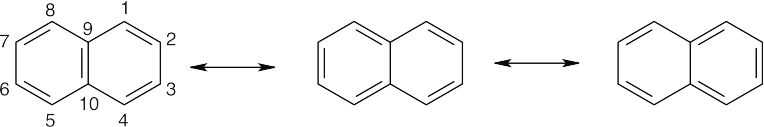
The bond between carbons 1 and 2 is represented as a double bond in two of the three resonance structures, but the bond between carbons 2 and 3 is represented as a double bond in only one resonance structure. The C1–C2 bond thus has more double-bond character in the resonance hybrid, and it is shorter than the C2–C3 bond. The C3–C4, C5–C6, and C7–C8 bonds also have more double-bond character than the remaining bonds. |
| 8.27 |  |
| 8.28-8.29 | 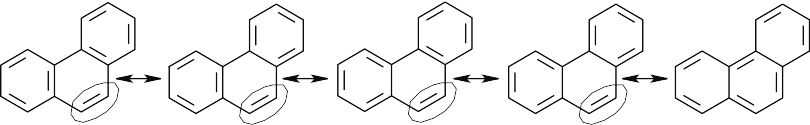
The circled bond is represented as a double bond in four of the five resonance forms of phenanthrene. This bond has more double-bond character and thus is shorter than the other carbon–carbon bonds of phenanthrene. |
| 8.30 | 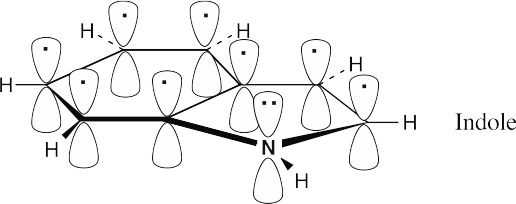
Indole, like naphthalene, has ten π electrons in two rings and is aromatic. Two π electrons come from the nitrogen atom. |
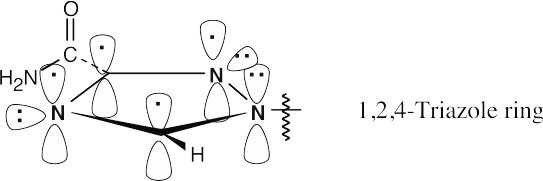
| 8.31 | The 1,2,4-triazole ring is aromatic because it has 6 π electrons in a cyclic, conjugated system.
|
Mechanisms of Electrophilic Substitutions
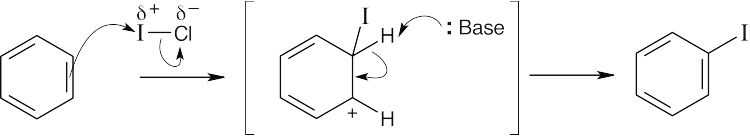
| 8.32 |
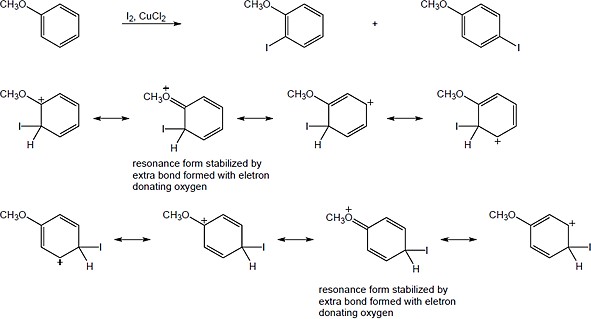
ICl can be represented as Iδ+ — Clδ− because chlorine is a more electronegative element than iodine. Iodine can act as an electrophile in electrophilic aromatic substitution reactions. |
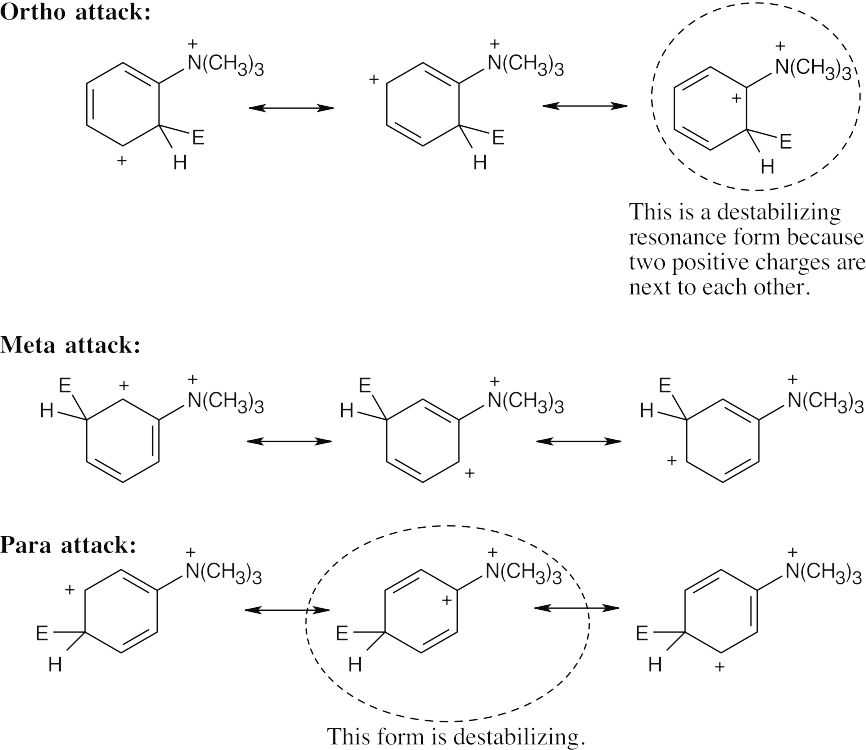
| 8.33 | When an electrophile reacts with an aromatic ring bearing a (CH3)3N+– group:
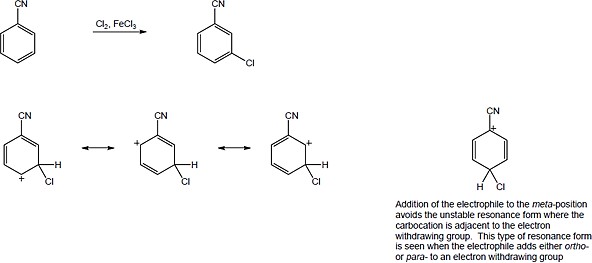
The N,N,N-trimethylammonium group has no electron-withdrawing resonance effect because it has no vacant p orbitals to overlap with the π orbital system of the aromatic ring. The (CH3)3N+– group is inductively deactivating, however, because it is positively charged. It is meta-directing because the cationic intermediate resulting from meta attack is somewhat more stable than those resulting from ortho or para attack. |
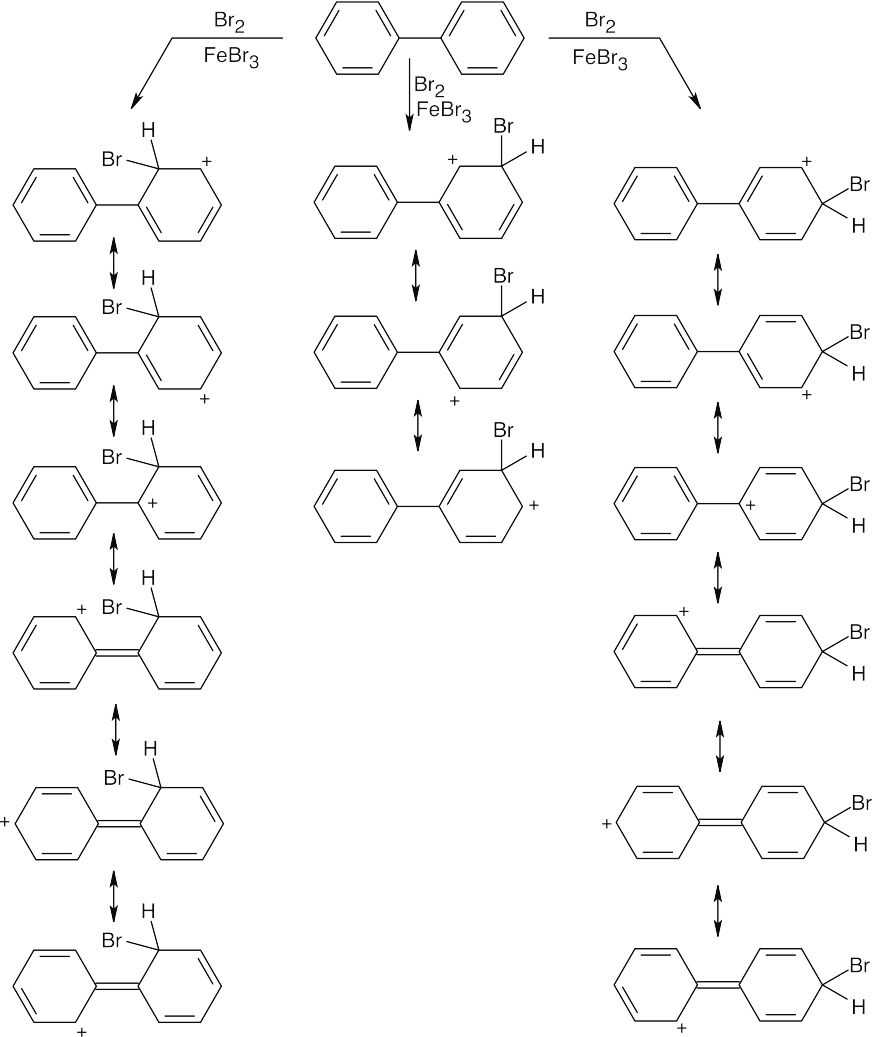
| 8.34 |
Resonance structures show that bromination occurs in the ortho and para positions of the rings. The positively charged intermediate formed from ortho or para attack can be stabilized by resonance contributions from the second ring of biphenyl, but this stabilization is not possible for meta attack. |
| 8.35 |
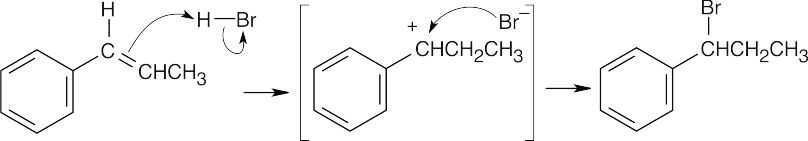
Protonation of the double bond at carbon 2 of 1-phenylpropene leads to an intermediate that can be stabilized by resonance involving the benzene ring. |
| 8.36 |
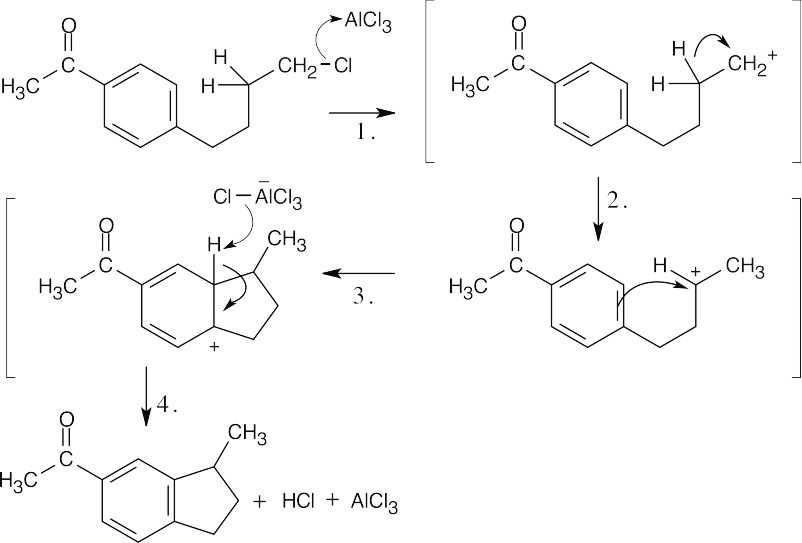
Step 1: Formation of primary carbocation. Step 2: Rearrangement to a secondary carbocation. Step 3: Attack of ring π electrons on the carbocation. Step 4: Loss of H+. This reaction takes place despite the fact that an electron-withdrawing group is attached to the ring. Apparently, the cyclization reaction is strongly favored. |
| 8.37 |
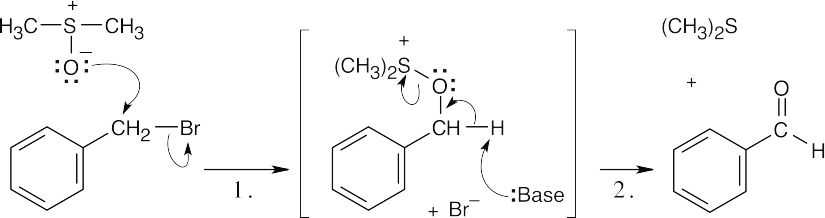
Step 1: SN2 displacement takes place when the negatively charged oxygen of dimethyl sulfoxide attacks the benzylic carbon of benzyl bromide, displacing Br–. Step 2: Base removes a benzylic proton, and dimethyl sulfide is eliminated in an E2 reaction. |
Reactivity and Orientation of Electrophilic Substitutions
| 8.38 |
|
| 8.39 |
Only methoxybenzene reacts faster than benzene (See Figure 8.11). |
| 8.40 | Most reactive ———–> Least reactive
|
| 8.41 |
|
| 8.42 | Most reactive ——–> Least reactive
Phenol > Toluene > p-Bromotoluene > Bromobenzene Aniline and nitrobenzene don’t undergo Friedel–Crafts alkylations. |
| 8.43 |
|
| 8.44 |
|
Organic Synthesis
| 8.45 |
|
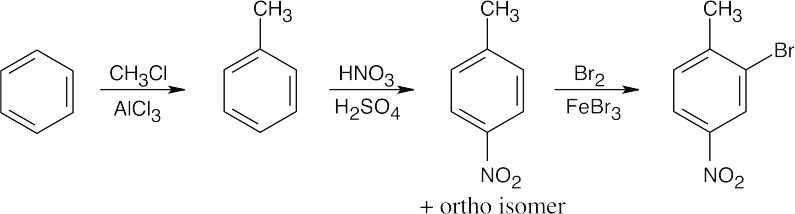
| 8.46 | When synthesizing substituted aromatic rings, it is necessary to introduce substituents in the proper order. A group that is introduced out of order will not have the proper directing effect. Remember that in many of these reactions a mixture of ortho and para isomers may be formed.
|
General Problems
| 8.47 | 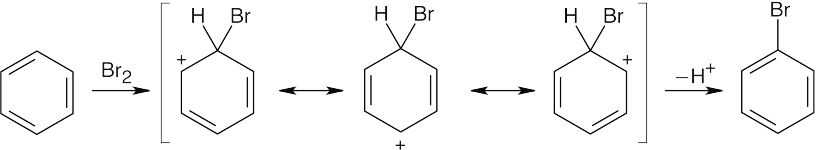 |
| 8.48 | 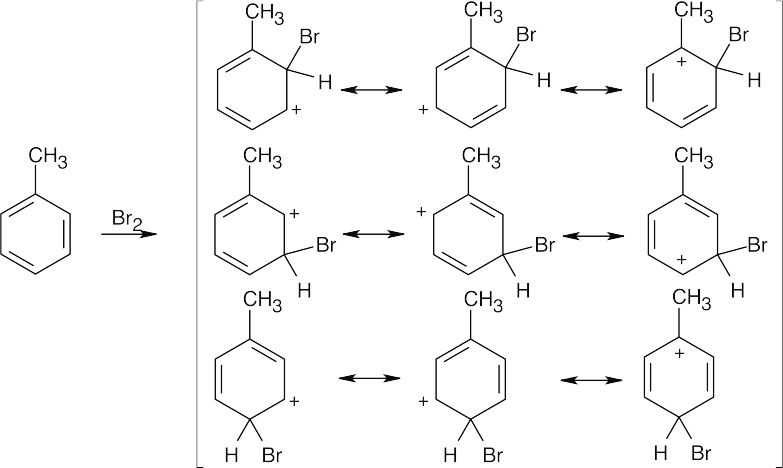
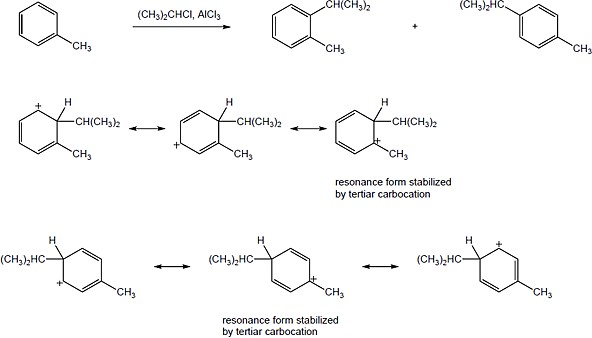
The ortho and para products predominate because the intermediate carbocation is more stabilized. The third resonance form drawn for ortho-para attack places the positive charge at the methyl-substituted carbon, which is a more stable tertiary carbocation. |
| 8.49 | Note: In the cyclic compounds, the charge can be placed on every atom in the ring, while in the linear compounds the charge can only reside on every other atom. | |
| (a) | 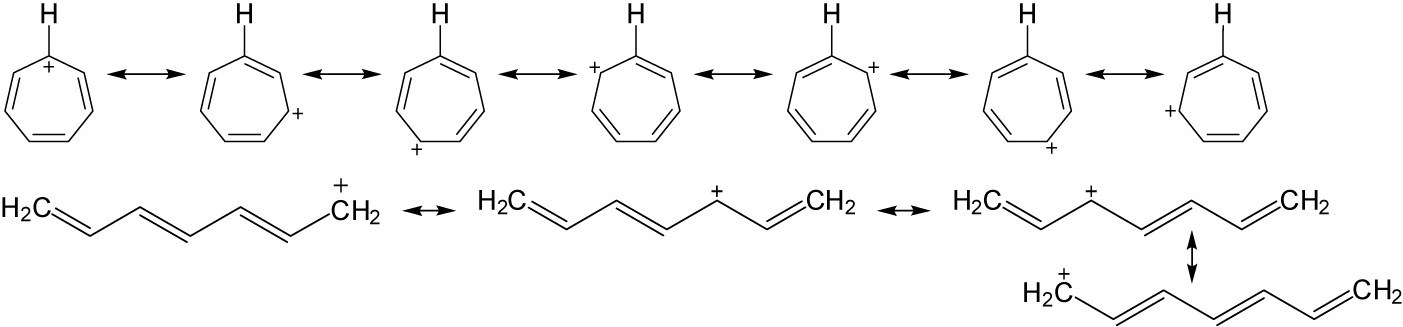 |
|
| (b) | 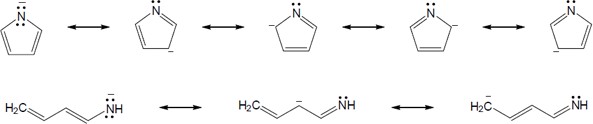 |
|
| 8.50 | (a) | 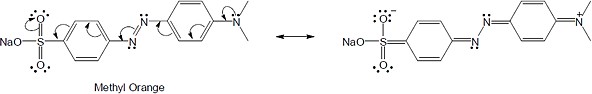 |
| (b) | 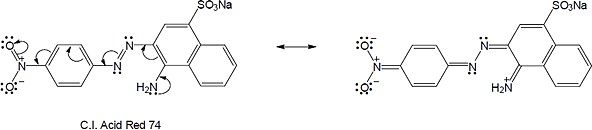 |
| 8.51 | Resonance forms for the intermediate from attack at C1:
|
| 8.52 | Both of these syntheses test your ability to carry out steps in the correct order.
|
| 8.53 | Problem 8.35 shows the mechanism of the addition of HBr to 1-phenylpropene and shows how the aromatic ring stabilizes the carbocation intermediate. For the methoxyl-substituted styrene, an additional resonance form can be drawn in which the cation is stabilized by the electron-donating resonance effect of the oxygen atom. For the nitro-substituted styrene, the cation is destabilized by the electron-withdrawing effect of the nitro group.
Thus, the intermediate resulting from addition of HBr to the methoxyl-substituted styrene is more stable, and reaction of p-methoxystyrene is faster. |
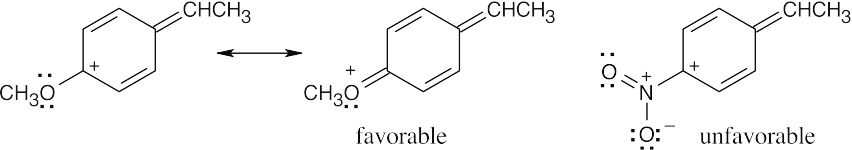
| 8.54 |
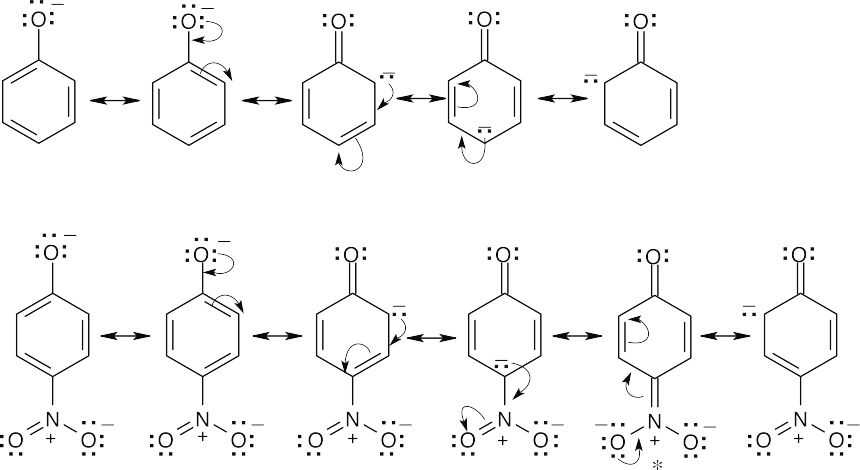
An electron-withdrawing substituent destabilizes a positively charged intermediate (as in electrophilic aromatic substitution) but stabilizes a negatively charged intermediate. For the dissociation of a phenol, an –NO2 group stabilizes the phenoxide anion by resonance, thus lowering ΔG° and pKa. In the starred resonance form for p-nitrophenol, the negative charge has been delocalized onto the oxygens of the nitro group. |
| 8.55 | For the same reason described in the previous problem, a methyl group destabilizes the negatively charged intermediate, thus raising ΔG° and pKa, making this phenol less acidic. |
| 8.56 |
|