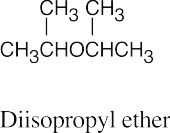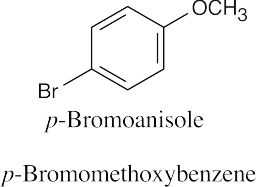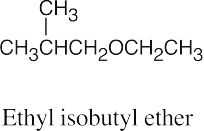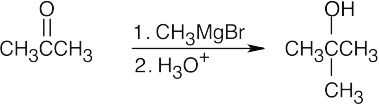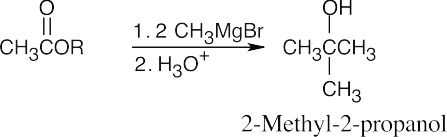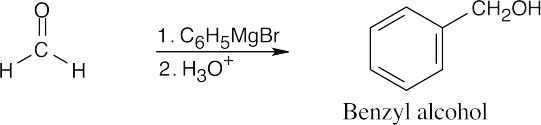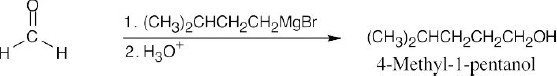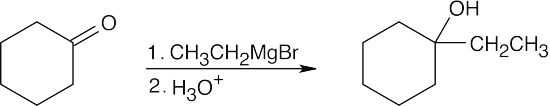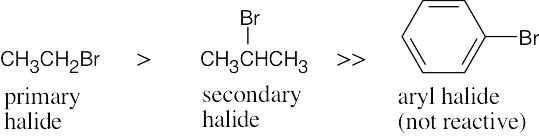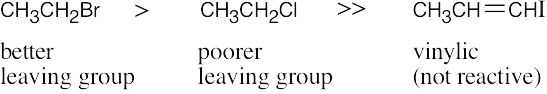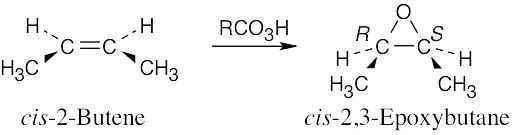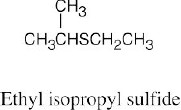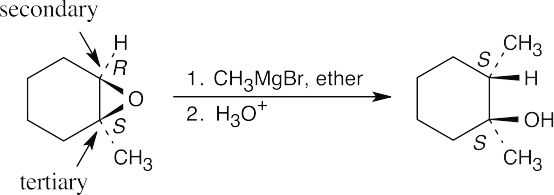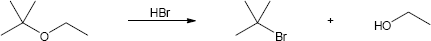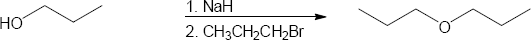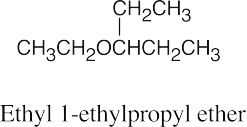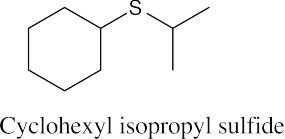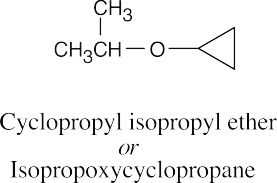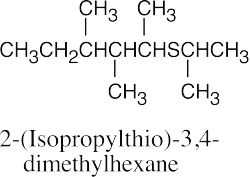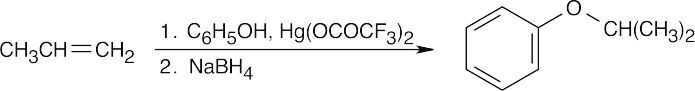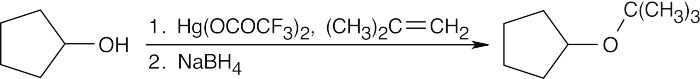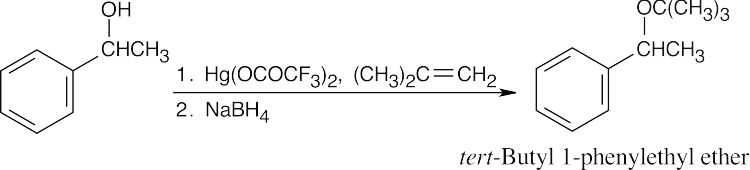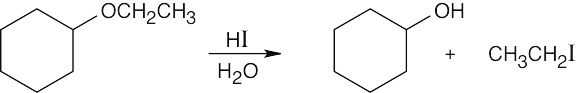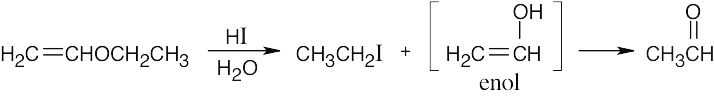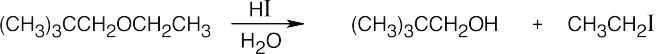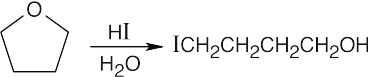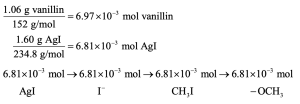9 Chapter 9 Solutions to Problems – Alcohols, Phenols, Ethers, Thiols, Sulfides
Chapter 9 – Alcohols, Phenols, Ethers, Thiols, Sulfides
Solutions to Problems
| 9.1 | The parent chain must contain the hydroxyl group, and the hydroxyl group(s) should receive the lowest possible number. | |||
| (a) | 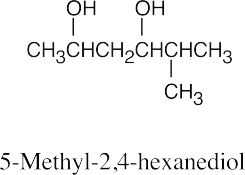 |
(b) | 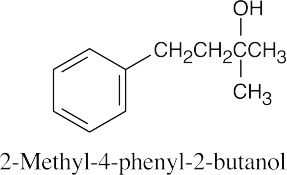 |
|
| (c) | 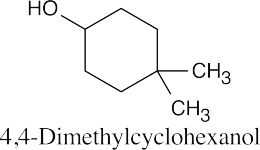 |
(d) | 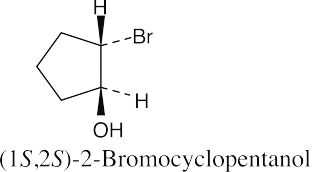 |
|
| (e) | 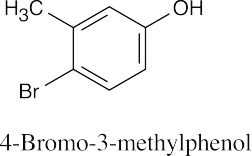 |
(f) | 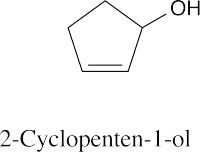 |
|
| 9.2 | (a) | 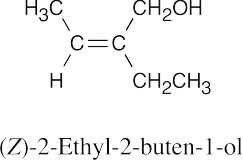 |
(b) | 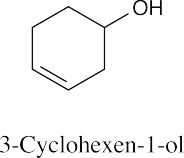 |
| (c) | 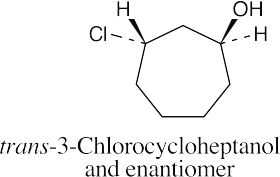 |
(d) | 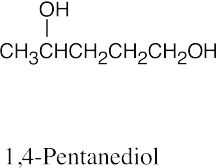 |
|
| (e) | 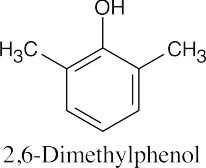 |
(f) | 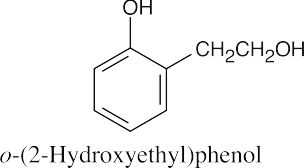 |
| 9.3 | In general, the boiling points of a series of isomers decrease with branching. The more nearly spherical a compound becomes, the less surface area it has relative to a straight chain compound of the same molecular weight and functional group type. A smaller surface area allows fewer van der Waals interactions, the weak forces that cause covalent molecules to be attracted to each other.
In addition, branching in alcohols makes it more difficult for hydroxyl groups to approach each other to form hydrogen bonds. A given volume of 2-methyl-2-propanol therefore contains fewer hydrogen bonds than the same volume of 1-butanol, and less energy is needed to break them in boiling. |
| 9.4 |  |
||||
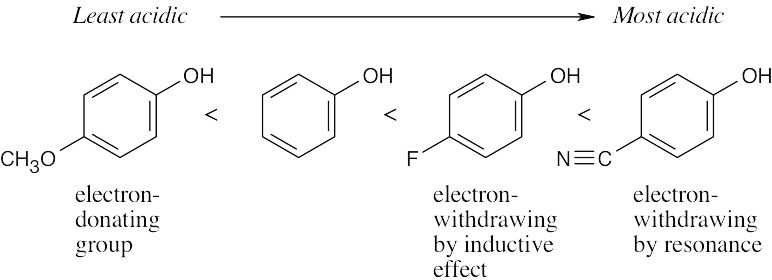
| (a) | HC≡CH < | (CH3)2CHOH< | CH3OH < | (CF3)2CHOH | |
| alkyne | hindered alcohol | alcohol | alcohol with electron-withdrawing groups | ||
| (b) | p-Methylphenol < | Phenol < | p-(Trifluoromethyl)phenol | ||
| phenol with electron-donating groups | phenol with electron-withdrawing groups | ||||
| 9.5 | Ethers can be named either as alkoxy-substituted compounds or by citing the two groups bonded to oxygen, followed by the word “ether”.
|
| 9.6 | (a) | 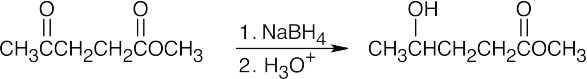
NaBH4 reduces aldehydes and ketones without interfering with other functional groups. |
| (b) | 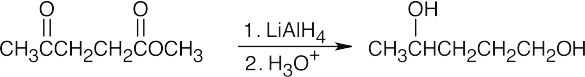
LiAlH4, a stronger reducing agent, reduces both ketones and esters. |
|
| (c) | 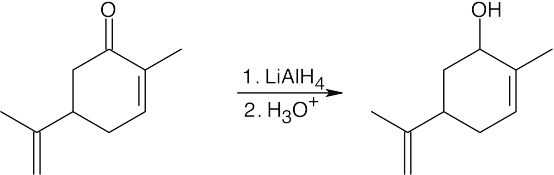
LiAlH4 reduces carbonyl functional groups without reducing double bonds. |
| 9.7 | (a) | 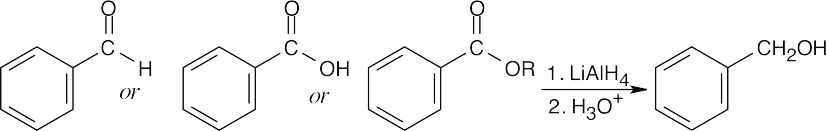
Benzyl alcohol may be the reduction product of an aldehyde, a carboxylic acid, or an ester. NaBH4 may be used to reduce the aldehyde. |
| (b) | 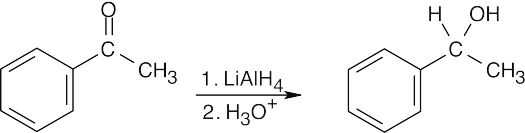
Reduction of a ketone yields the secondary alcohol. NaBH4 may also be used here and in (c). |
|
| (c) | 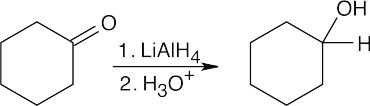 |
|
| (d) |  |
| 9.8 | All of the products have an –OH and a methyl group bonded to what was formerly a ketone carbon. | |
| (a) | 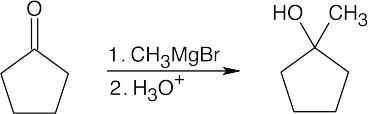 |
|
| (b) | 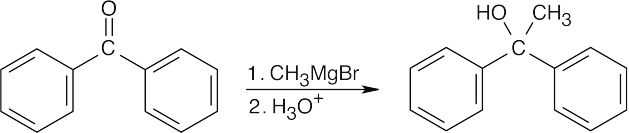 |
|
| (c) | 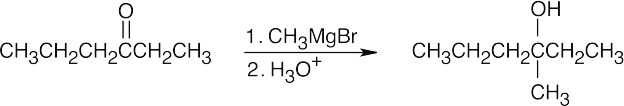 |
|
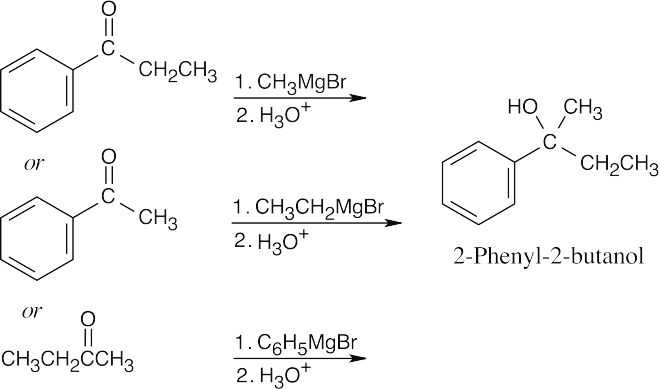
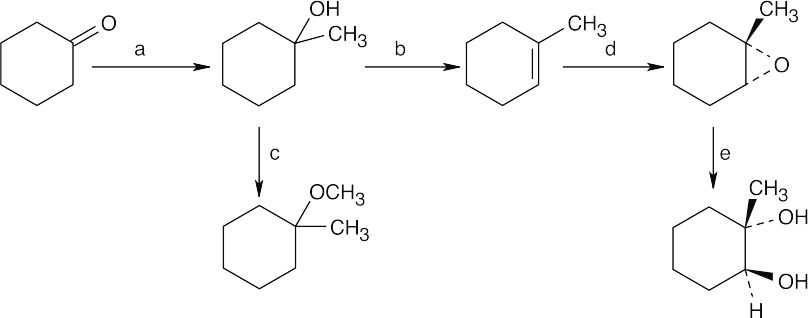
| 9.9 | First, identify the type of alcohol. If the alcohol is primary, it can only be synthesized from formaldehyde plus the appropriate Grignard reagent. If the alcohol is secondary, it is synthesized from an aldehyde and a Grignard reagent. (Usually, there are two combinations of aldehyde and a Grignard reagent). A tertiary alcohol is synthesized from a ketone and a Grignard reagent. If all three groups on the tertiary alcohol are different, there are often three different combinations of ketone and a Grignard reagent. If two of the groups on the alcohol carbon are the same, the alcohol may also be synthesized from an ester and two equivalents of Grignard reagents. | |
| (a) | 2-Methyl-2-propanol is a tertiary alcohol. To synthesize a tertiary alcohol, start with a ketone.
If two or more alkyl groups bonded to the carbon bearing the –OH group are the same, an alcohol can be synthesized from an ester and a Grignard reagent.
|
|
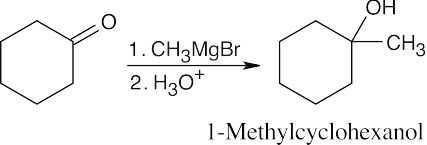
| (b) | Since 1-methylcyclohexanol is a tertiary alcohol, start with a ketone.
|
|
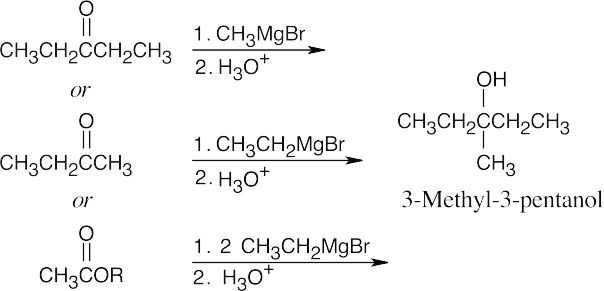
| (c) | 3-Methyl-3-pentanol is a tertiary alcohol. When two of the three groups bonded to the alcohol carbon are the same, either a ketone or an ester can be used as a starting material.
|
|
| (d) | Three possible combinations of ketone plus a Grignard reagent can be used to synthesize this tertiary alcohol.
|
|
| (e) | Formaldehyde must be used to synthesize this primary alcohol.
|
|
| (f) | As in (e), use formaldehyde to synthesize a primary alcohol.
|
|
| 9.10 | First, interpret the structure of the alcohol. This alcohol, 1-ethylcyclohexanol, is a tertiary alcohol that can be synthesized from a ketone. Only one combination of ketone and a Grignard reagent is possible.
|
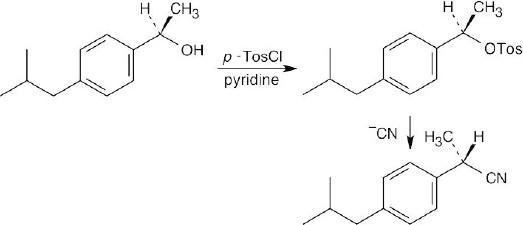
| 9.11 | Recall from Chapter 11 that –OH is a very poor leaving group in reactions run under SN2 conditions. A toluenesulfonate, however, is a very good leaving group, and reaction of the toluenesulfonate of the alcohol with –CN proceeds readily under SN2 conditions to give the desired product with inversion of configuration at the chirality center.
|
| 9.12 | Aldehydes are synthesized from oxidation of primary alcohols, and ketones are synthesized from oxidation of secondary alcohols. | |
| (a) | 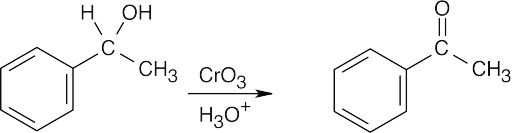 |
|
| (b) | 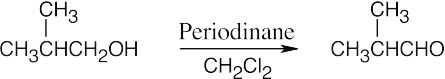 |
|
| (c) | 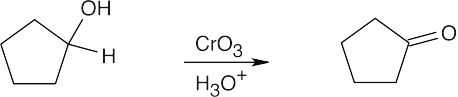 |
|
| 9.13 | (a) | |
| (b) |
| 9.14 | The first step of the dehydration mechanism is protonation of an alcohol. Water is then displaced by another molecule of alcohol to form an ether. If two different alcohols are present, either one can be protonated and either one can displace water, yielding a mixture of products.
If this procedure were used with ethanol and 1-propanol, the products would be diethyl ether, ethyl propyl ether, and dipropyl ether. If there were equimolar amounts of the alcohols, and if they were of equal reactivity, the product ratio would be diethyl ether: ethyl propyl ether: dipropyl ether = 1:2:1. |
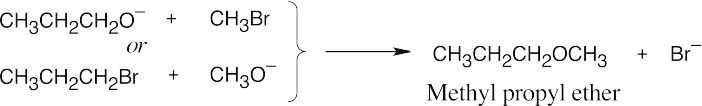
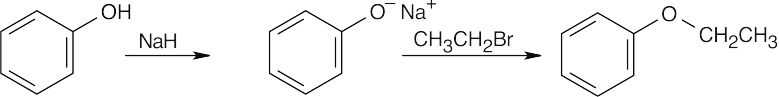
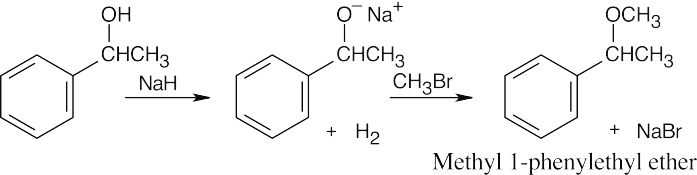
| 9.15 | Remember that the halide in the Williamson ether synthesis should be primary or methyl, in order to avoid competing elimination reactions. The alkoxide anions shown are formed by treating the corresponding alcohols with NaH.
|
| 9.16 | The compounds most reactive in the Williamson ether synthesis are also most reactive in any SN2 reaction (review Chapter 7 if necessary).
Most reactive ———–> Least reactive
|
| 9.17 |
|
| 9.18 |
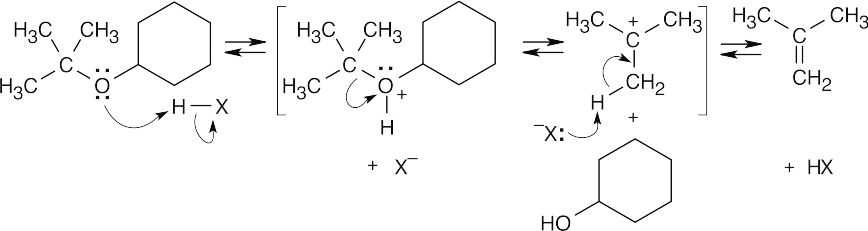
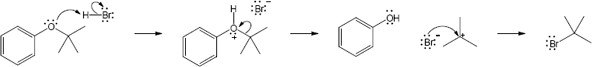
The first step of acid-catalyzed ether cleavage is protonation of the ether oxygen to give an intermediate oxonium ion, which collapses to form an alcohol and a tertiary carbocation. The carbocation then loses a proton to form an alkene, 2-methylpropene. This is an example of E1 elimination. The acid used for cleavage is often trifluoroacetic acid. |
| 9.19 |
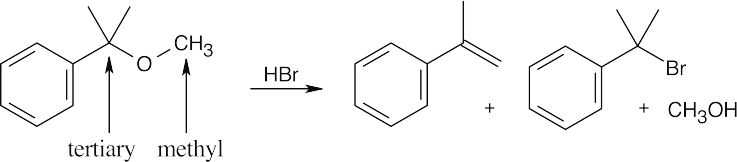
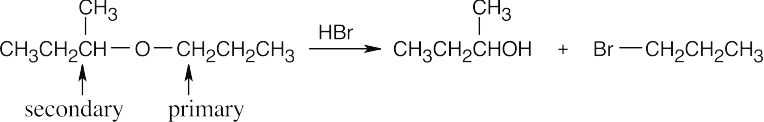
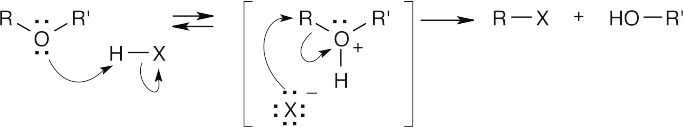
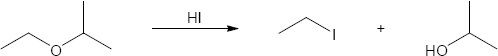
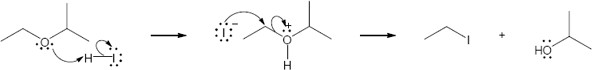
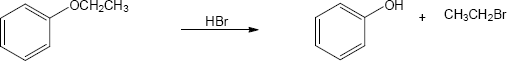
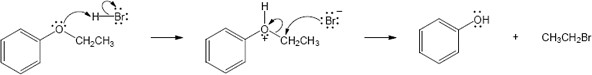
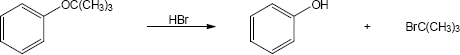
HX first protonates the oxygen atom, and halide then brings about a nucleophilic displacement to form an alcohol and an organic halide. The better the nucleophile, the more effective the displacement. Since I– and Br– are better nucleophiles than Cl–, ether cleavage proceeds more smoothly with HI or HBr than with HCl. |
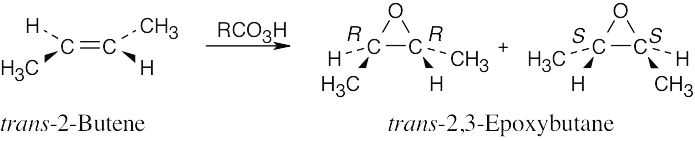
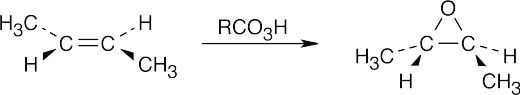
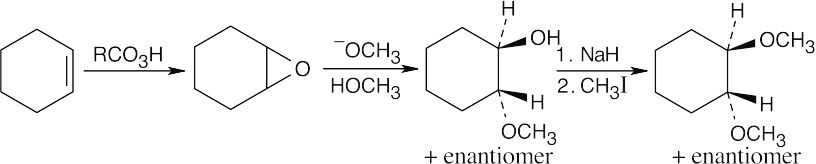
| 9.20-9.21 | Epoxidation by use of m-chloroperoxybenzoic acid (RCO3H) is a syn addition of oxygen to a double bond. The original bond stereochemistry is retained, and the product is a meso compound.
|
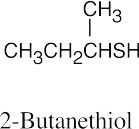
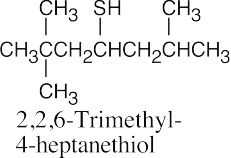
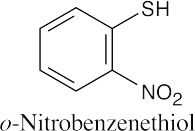
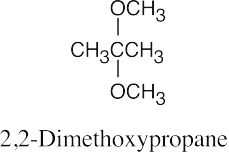
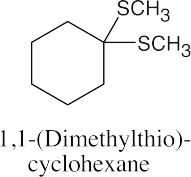
| 9.22 | Thiols are named by the same rules as alcohols, with the suffix -ol replaced by the suffix-thiol. Sulfides are named by the same rules as ethers, with “sulfide” replacing “ether”.
|
Additional Problems
Visualizing Chemistry
| 9.23 | (a) | 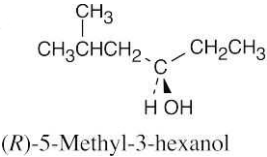 |
(b) | 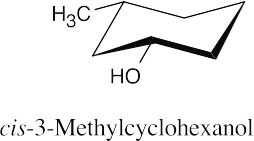 |
| (c) |  |
(d) | 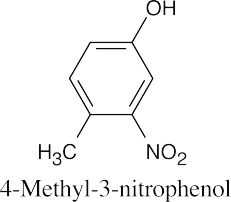 |
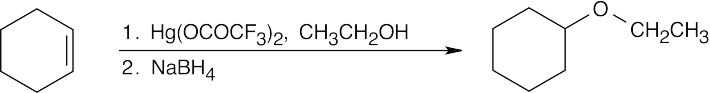
| 9.24 | The reduction product is a racemic mixture. Reaction of the (S) enantiomer is shown. | ||
| (a) | 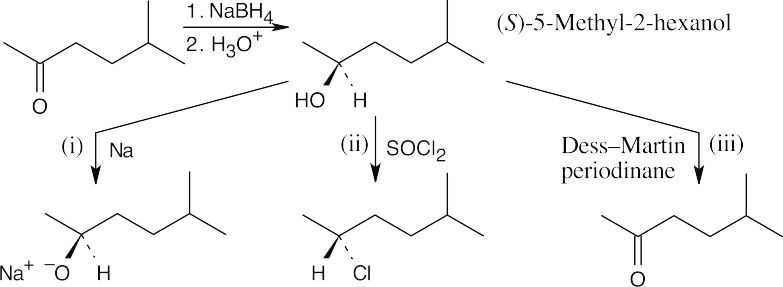 |
||
| (b) | 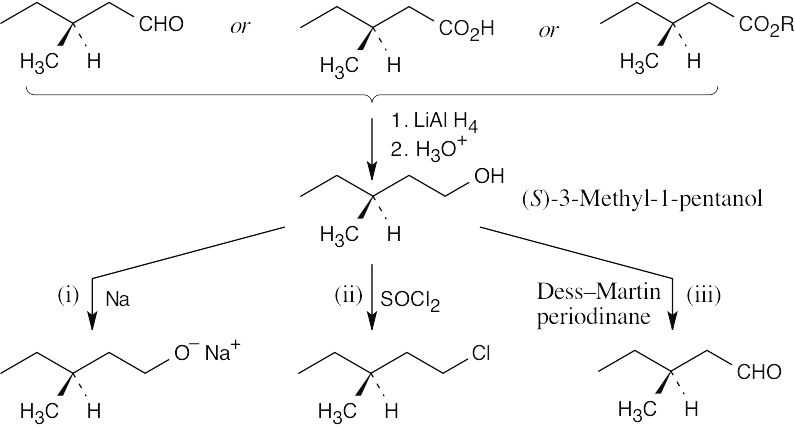 |
||
| 9.25 | (a) | 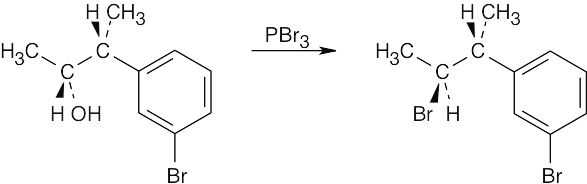 |
| (b) | 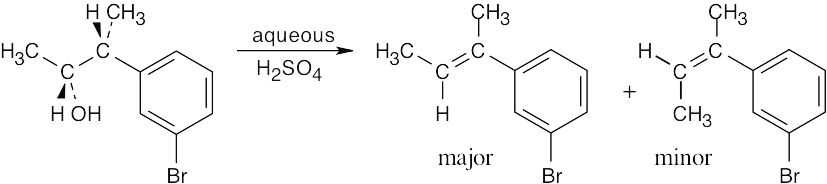 |
|
| (c) | 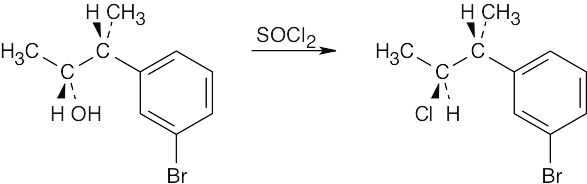 |
|
| (d) | 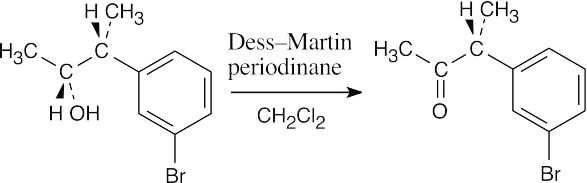 |
| 9.26 | (a) | 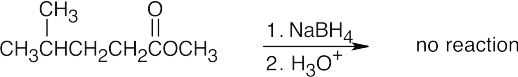 |
| (b) | 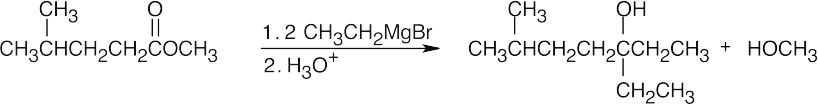 |
| 9.27 | 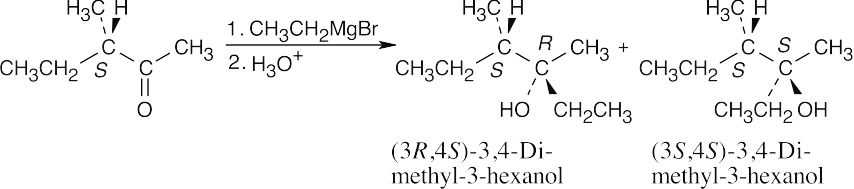
The product is a mixture of the (3R,4S) and (3S,4S) diastereomers. The diastereomers are formed in unequal amounts, and the product mixture is optically active. We can’t predict which diastereomer will predominate. |
| 9.28 |
|
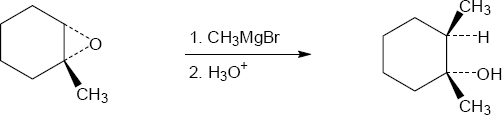
| 9.29 | The Grignard reagent attacks the epoxide at the less hindered carbon in an SN2 reaction. The oxygen remains bonded to the tertiary carbon.
|
Mechanism Problems
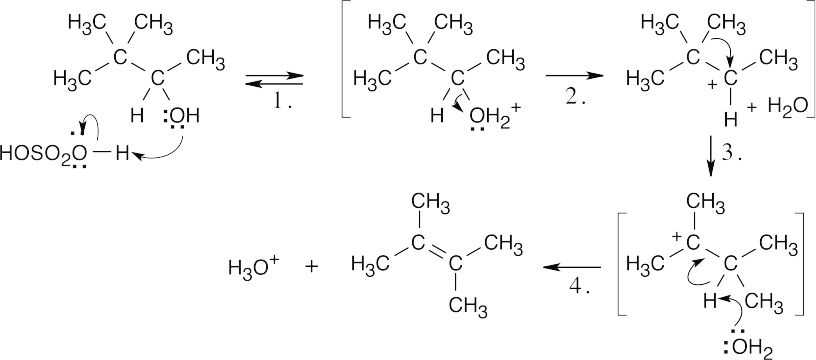
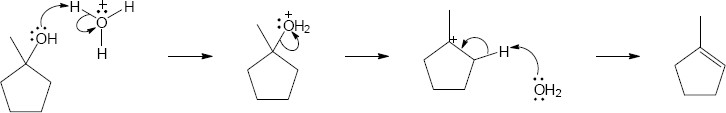
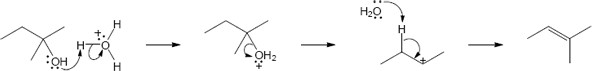
| 9.30 |
|
|
| Step 1: | Protonation. | |
| Step 2: | Loss of H2O. | |
| Step 3: | Alkyl shift to form the tertiary carbocation. | |
| Step 4: | Loss of H3O+. | |
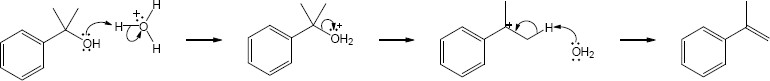
| 9.31 | 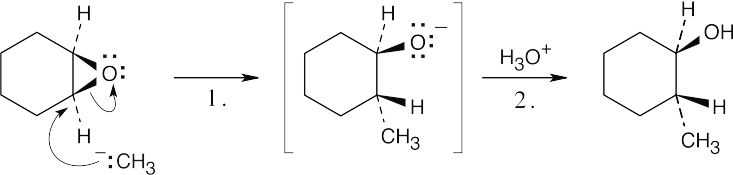 |
|
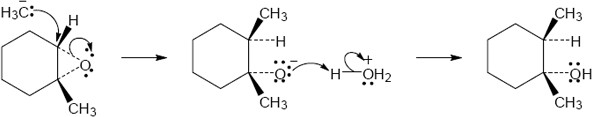
| Step 1: | SN2 reaction of Grignard reagent. | |
| Step 2: | Protonation of alkoxide oxygen. | |
| The methyl group and the hydroxyl group have a trans relationship. | ||
| 9.32 | 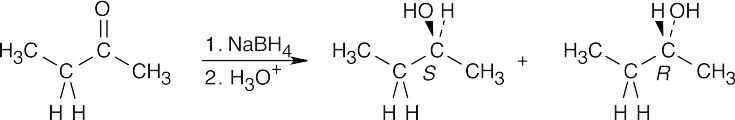
Reaction of 2-butanone with NaBH4 produces a racemic mixture of (R)-2-butanol and (S)-2-butanol. |
| 9.33 | (a) | 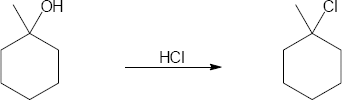
Mechanism:
|
| (b) | 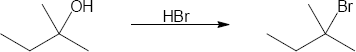
Mechanism:
|
|
| (c) | 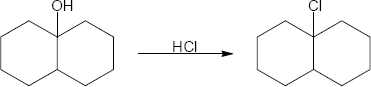
Mechanism:
|
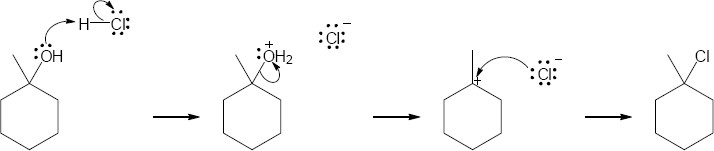
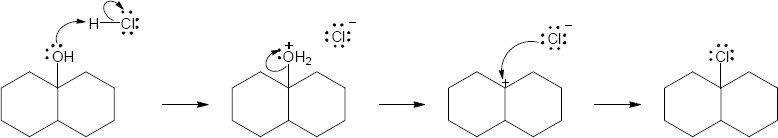
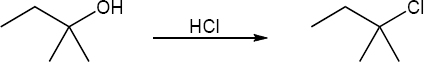
| 9.34 | (a) | Teritary alcohols undergo exclusively SN1 substitution.
|
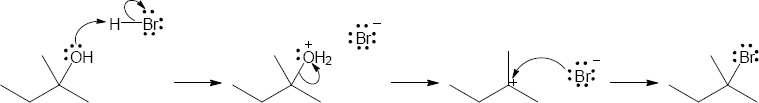
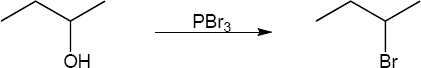
| (b) | Reactions with PBr3 undergo SN2 substitution
|
| 9.35 | (a) | 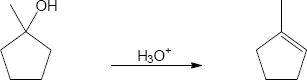
Mechanism:
|
| (b) | 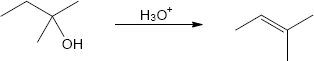
Mechanism:
|
|
| (c) | 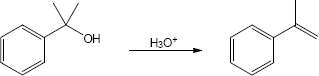
Mechanism:
|
| 9.36 | (a) | 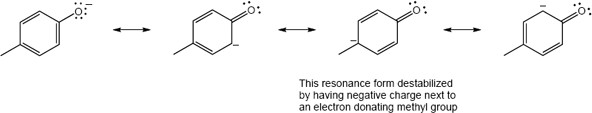 |
| (b) | 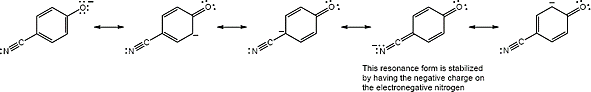 |
|
| (c) | 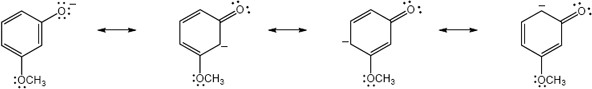
Because there is no direct effect through resonance, the OCH3 group does not have a significant effect on the pKa of the parent phenol |
| 9.37 |
|
| 9.38 |
|
| 9.39 |
|
| 9.40 |
|
| 9.41 |
|
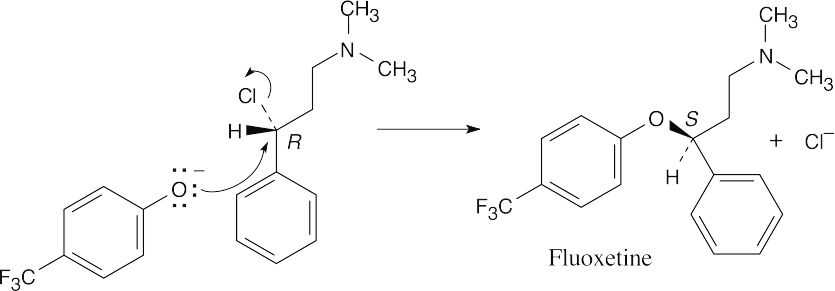
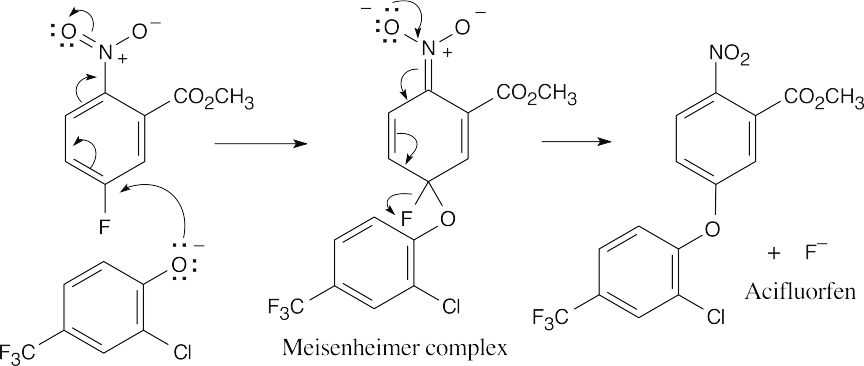
| 9.42 | The reaction is a nucleophilic aromatic substitution. The intermediate Meisenheimer complex is stabilized by the –NO2 group.
Step 1: Addition of phenoxide. Step 2: Elimination of fluoride. |
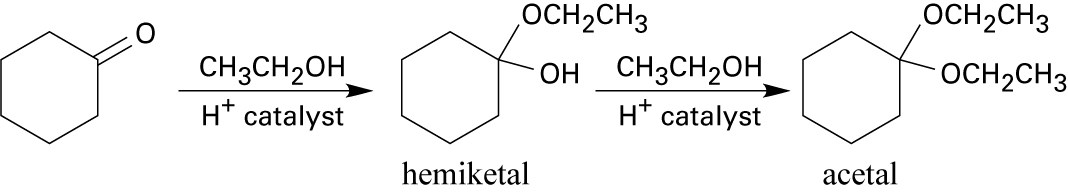
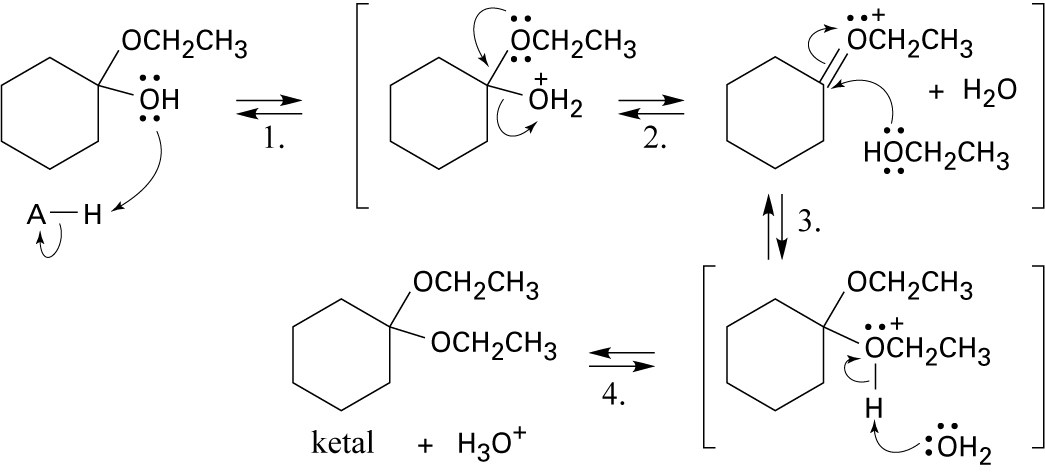
| 9.43 |
Step 1: Protonation. Step 2: Loss of water. Step 3: Addition of ethanol. Step 4: Loss of proton. |
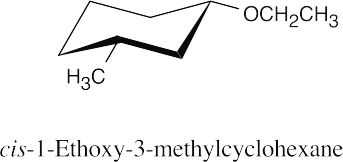
Naming Alcohols
| 9.44 | (a) | 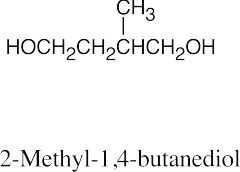 |
(b) | 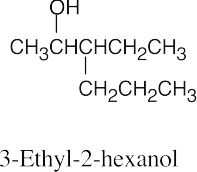 |
| (c) | 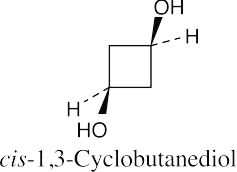 |
(d) | 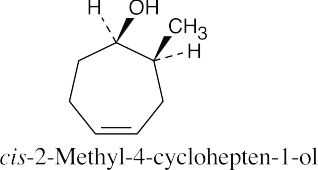 |
|
| (e) | 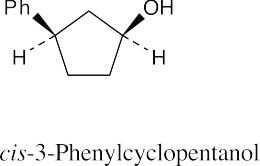 |
(f) | 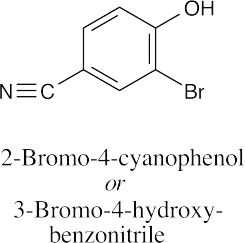 |
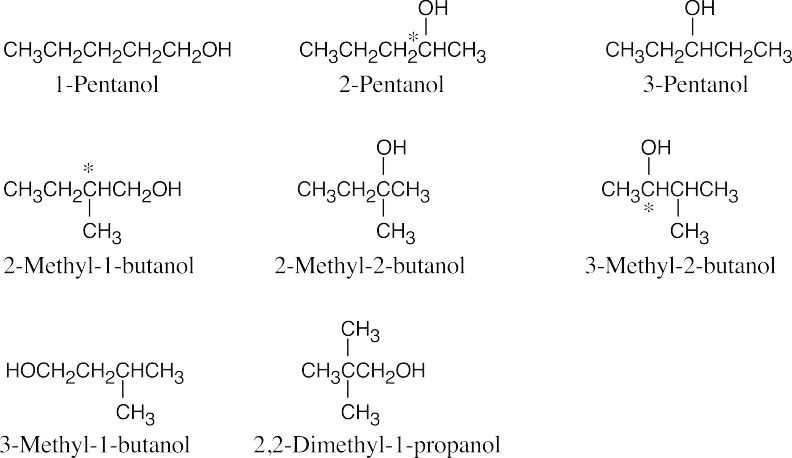
| 9.45 | None of these alcohols has multiple bonds or rings.
2-Pentanol, 2-methyl-1-butanol and 3-methyl-2-butanol have chiral carbons (starred). |
| 9.46 | (a) | 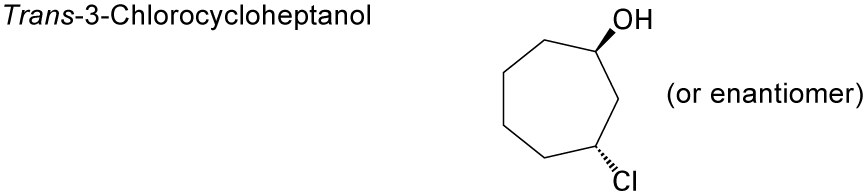 |
| (b) | 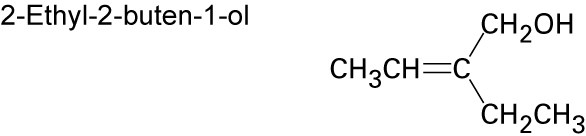 |
|
| (c) | 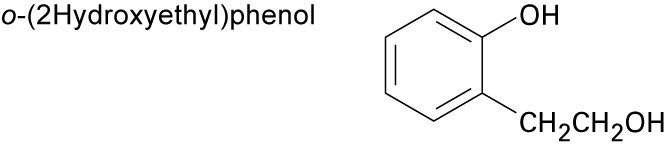 |
|
| (d) | 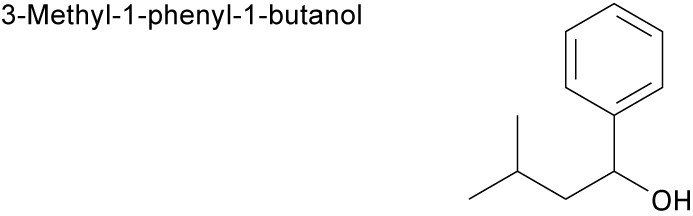 |
| 9.47 | 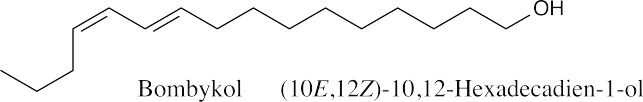 |
| 9.48 | 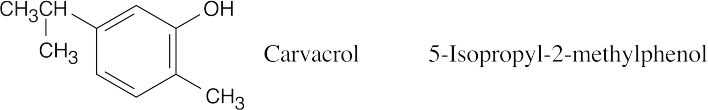 |
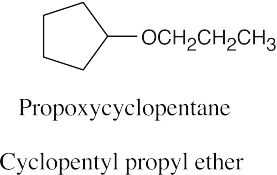
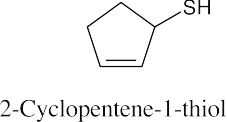
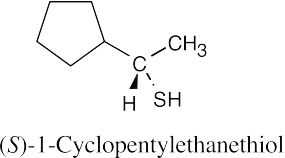
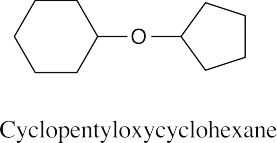
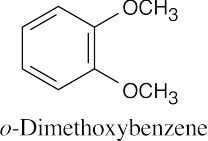
Naming Ethers, Thiols, and Sulfides
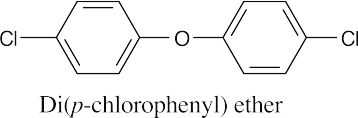
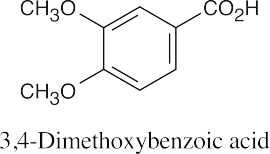
| 9.49 |
|
| 9.50 |
|
Synthesizing Alcohols
| 9.51 | In some of these problems, different combinations of a Grignard reagent and a carbonyl compound are possible. Remember that aqueous acid is added to the initial Grignard adduct to yield the alcohol. | |
| (a) | 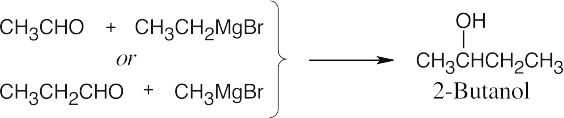 |
|
| (b) | 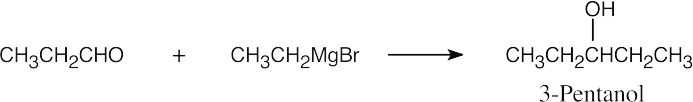 |
|
| (c) | 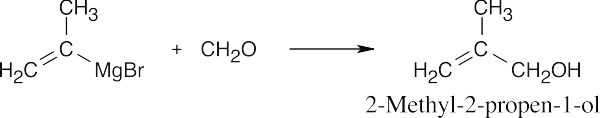 |
|
| (d) | 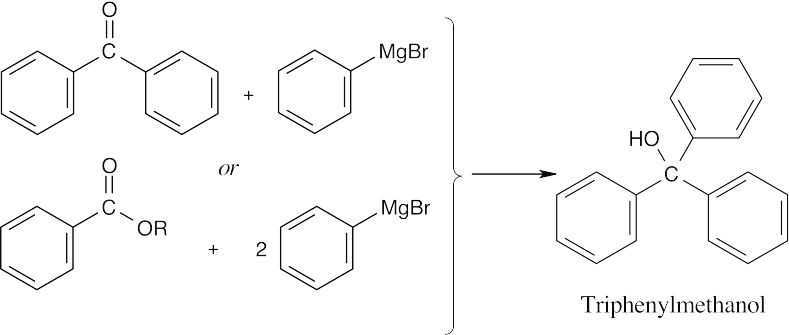 |
|
| (e) | 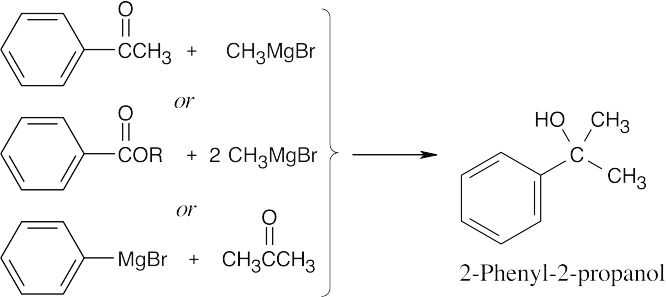 |
|
| (f) | 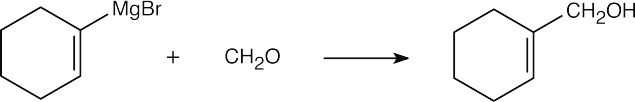 |
|
| 9.52 | (a) | 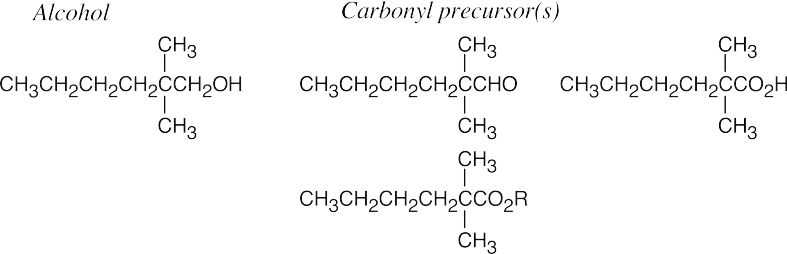 |
| (b) |  |
|
| (c) | 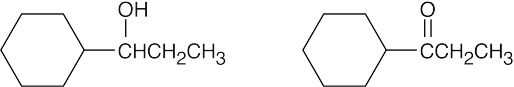 |
| 9.53 | Grignard Reagent + Carbonyl Compound ⎯⎯⎯⎯⎯⎯⎯⎯→ Product (after dilute acid workup) | |
| (a) | 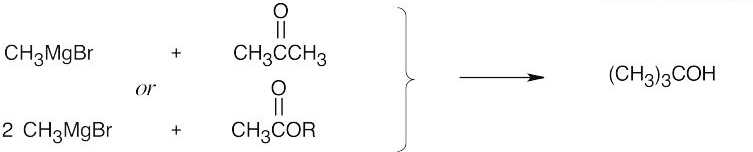 |
|
| (b) | 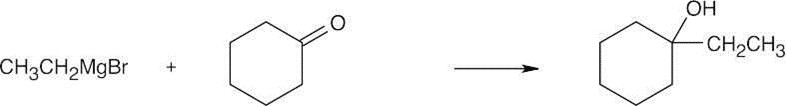 |
|
| (c) | 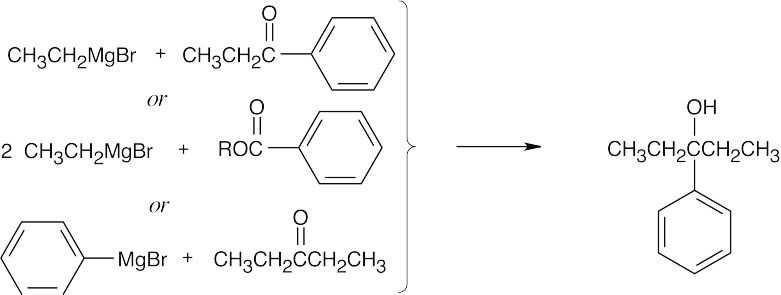 |
|
| (d) | 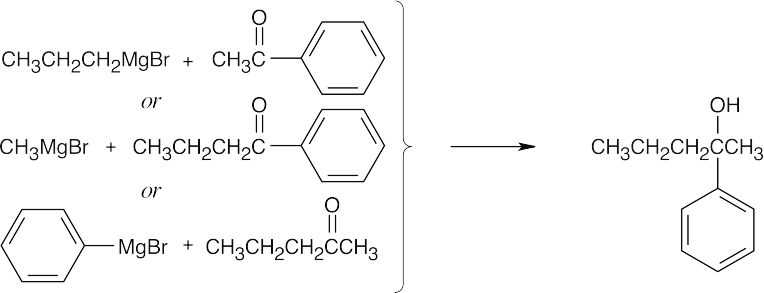 |
|
| (e) | 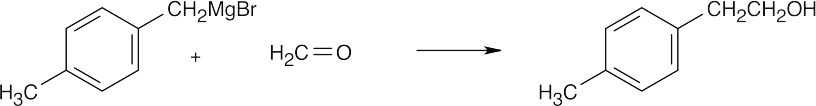 |
|
| (f) | 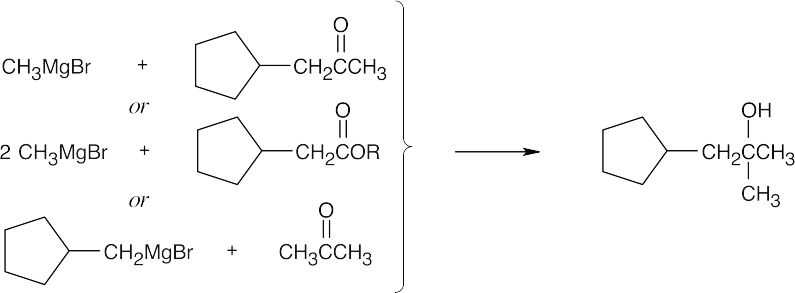 |
|
| 9.54 | All of these syntheses involve a Grignard reaction at some step. Both the carbonyl compound and the Grignard reagent must be prepared from alcohols. | |
| (a) | 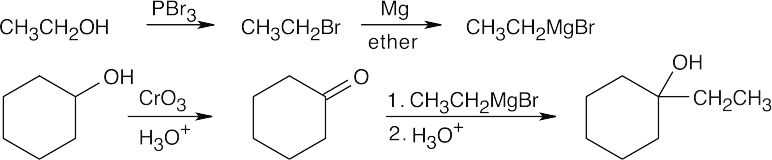 |
|
| (b) | 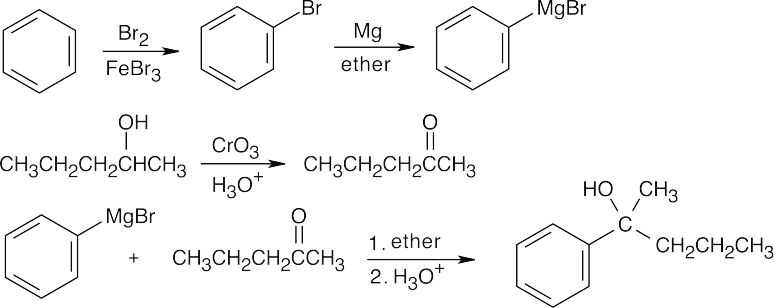 |
|
| (c) | 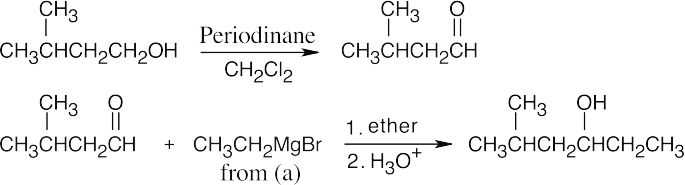 |
|
Reactions of Alcohols
| 9.55 | (a) | 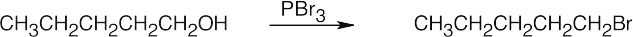 |
| (b) | 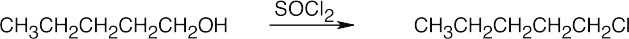 |
|
| (c) | 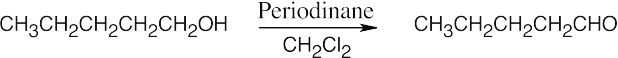 |
| 9.56 | (a) | 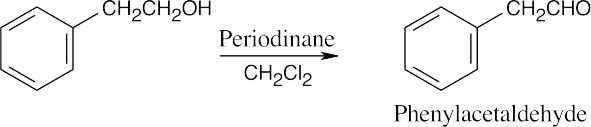 |
| (b) | 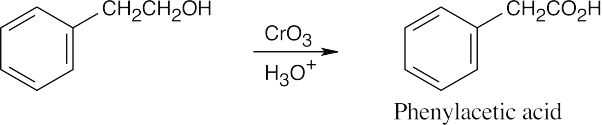 |
|
| (c) | 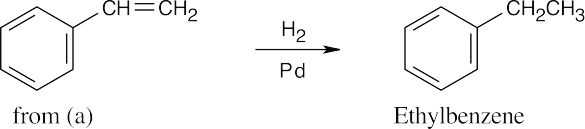 |
|
| (d) | 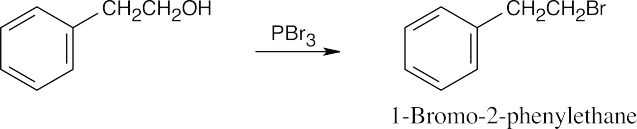 |
| 9.57 | 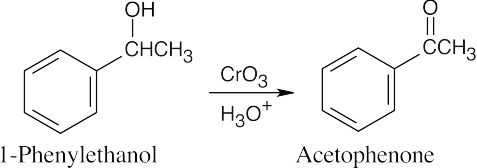 |
| 9.58 | (a) | 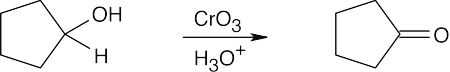 |
| (b) | 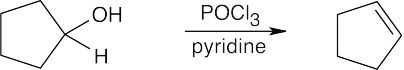 |
| 9.59 | (a) | 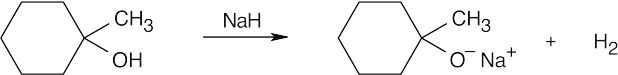 |
| (b) | 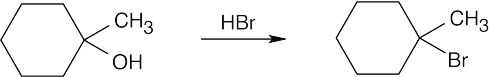 |
|
| (c) | 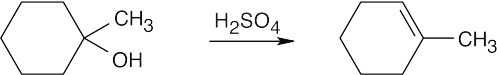 |
Synthesizing Ethers
| 9.60 |
|
| 9.61 |
|
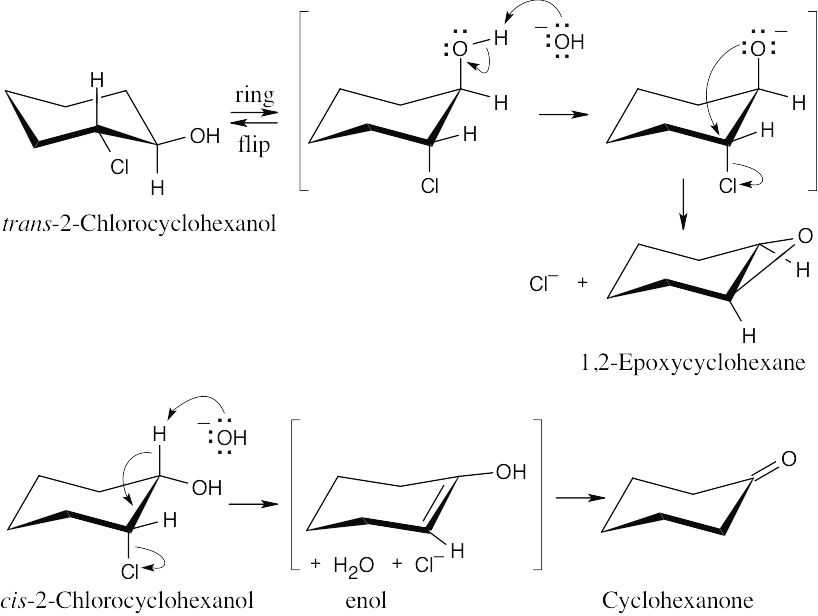
| 9.62 | In the trans isomer, the –OH and –Cl are in the trans orientation that allows epoxide formation to occur as described in Section 9.13. Epoxidation can’t occur for the cis isomer, however. Instead, the base –OH brings about E2 elimination, producing an enol, which tautomerizes to cyclohexanone.
|
Reactions of Ethers and Epoxides
| 9.63 |
|
| 9.64 |
|
| 9.65 |
|
| 9.66 |
The product of acid hydrolysis of cis-5,6-epoxydecane is a racemic mixture of R,R and S,S diols. |
| 9.67 |
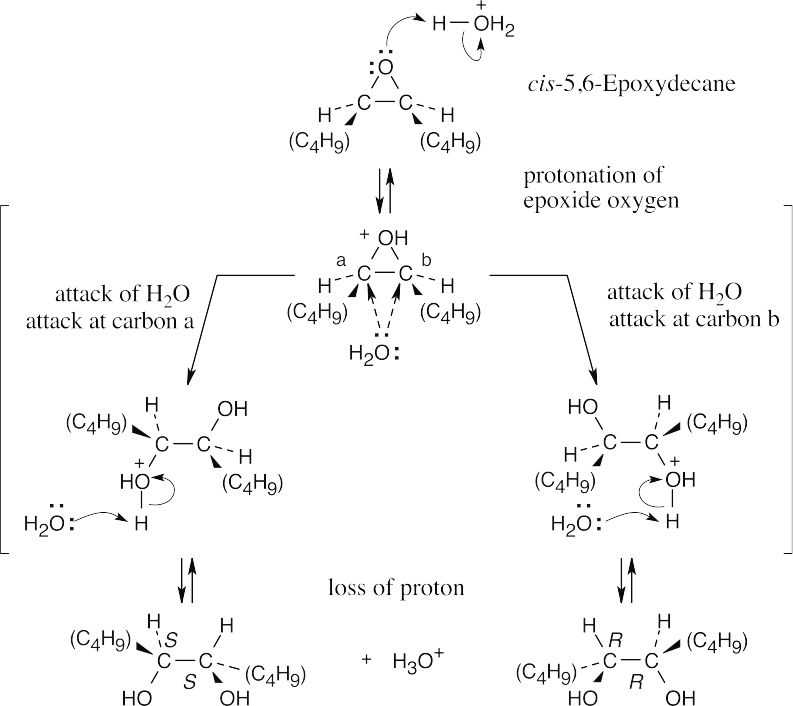
The product of acid hydrolysis of trans-5,6-epoxydecane is a meso compound that is a diastereomer of the products formed in the previous problem. |
| 9.68 |
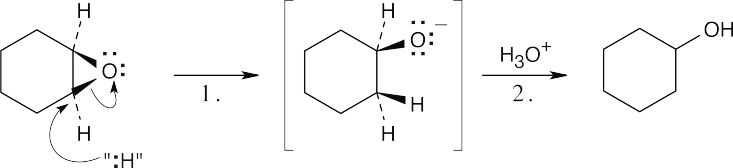
Step 1: Attack of the hydride nucleophile. Step 2: Protonation of the alkoxide anion. The reaction is an SN2 epoxide cleavage with “:H” as the nucleophile. The exact nature of the attacking nucleophile is not clear. |
General Problems
| 9.69 |
In these compounds you want to reduce some, but not all, of the functional groups present. To do this, choose the correct reducing agent. | |
| (a) | 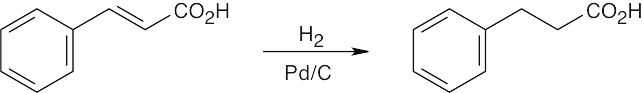
H2 with a palladium catalyst hydrogenates carbon–carbon double bonds without affecting carbonyl double bonds. |
|
| (b) | 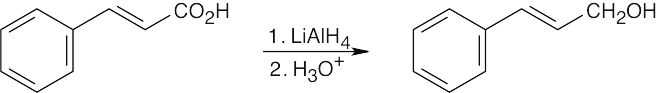
LiAlH4 reduces carbonyl groups without affecting carbon–carbon double bonds. |
|
| 9.70 | Remember that electron-withdrawing groups stabilize phenoxide anions and increase acidity. Electron-donating groups decrease phenol acidity.
|
| 9.71 | 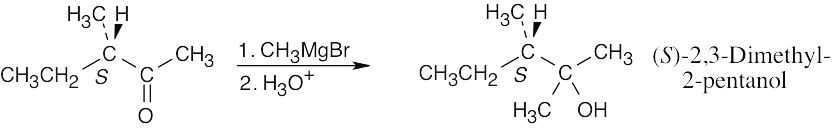
Despite this problem’s resemblance to Problem 17.24, the stereochemical outcome is different. Addition of methylmagnesium bromide to the carbonyl group doesn’t produce a new chirality center and doesn’t affect the chirality center already present. The product is pure (S)-2,3-dimethyl-2-pentanol, which is optically active. |
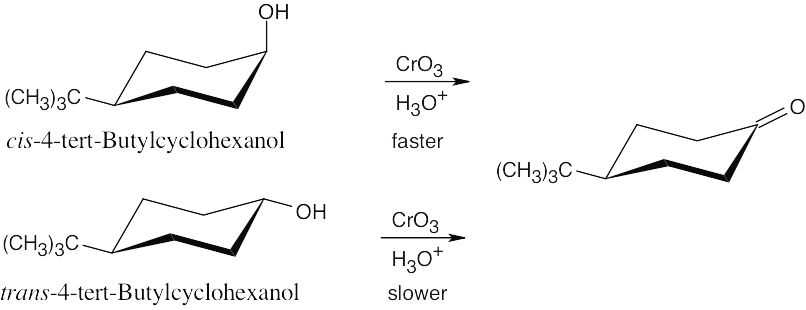
| 9.72 | The hydroxyl group is axial in the cis isomer, which is expected to oxidize faster than the trans isomer. (Remember that the bulky tert-butyl group is always equatorial in the more stable isomer.)
|
| 9.73 | An alcohol adds to an aldehyde by a mechanism that we will study in a later chapter. The hydroxyl group of the addition intermediate undergoes oxidation (as shown in Section 9.8), and an ester is formed.
|
| 9.74 | 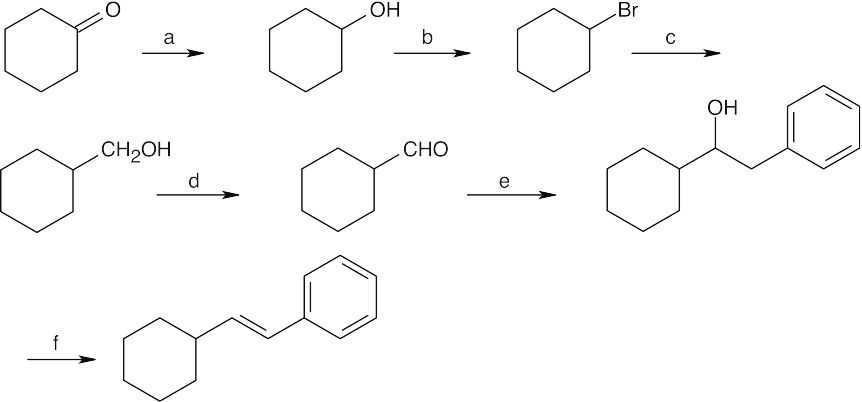 |
|||
| (a) | NaBH4, then H3O+ | (b) | PBr3 | |
| (c) | Mg, ether, then CH2O | (d) | Dess–Martin periodinane, CH2Cl2 | |
| (e) | C6H5CH2MgBr, then H3O+ | (f) | POCl3, pyridine | |
| 9.75 |
|
| 9.76 | The mechanism of Grignard addition to oxetane is the same as the mechanism of Grignard addition to epoxides, described in Section 9.14. The reaction proceeds at a reduced rate because oxetane is less reactive than ethylene oxide. The four-membered ring oxetane is less strained, and therefore more stable, than the three-membered ethylene oxide ring. |
| 9.77 |
|
| 9.78 |
(a) CH3MgBr, ether; (b) H2SO4, H2O; (c) NaH, then CH3I; (d) m-ClC6H4CO3H; (e) –OH, H2O. |