Visualizing Chemistry
Problem 8-22
Name the following alkenes, and predict the products of their reaction with (1) meta– chloroperoxybenzoic acid, (2) KMnO4 in aqueous acid, (3) O3, followed by Zn in acetic acid:
(a)
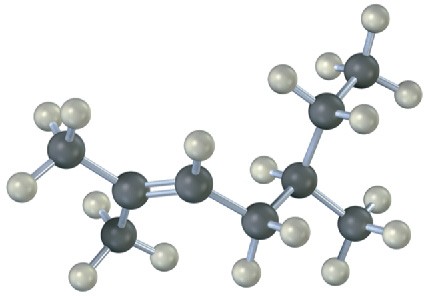
(b)
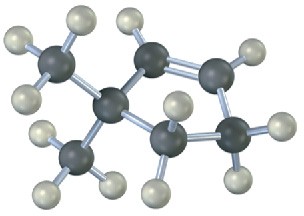
Problem 8-23
Draw the structures of alkenes that would yield the following alcohols on hydration (red = O). Tell in each case whether you would use hydroboration–oxidation or oxymercuration– demercuration.
(a)
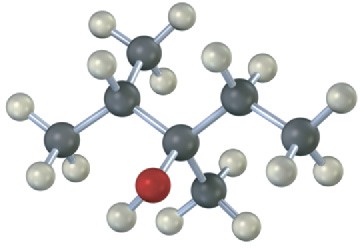
(b)
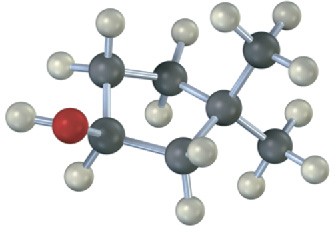
Problem 8-24
The following alkene undergoes hydroboration–oxidation to yield a single product rather than a mixture. Explain the result, and draw the product showing its stereochemistry.
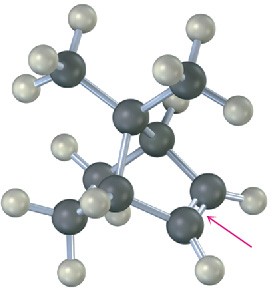
Problem 8-25
From what alkene was the following 1,2-diol made, and what method was used, epoxide hydrolysis or OsO4?
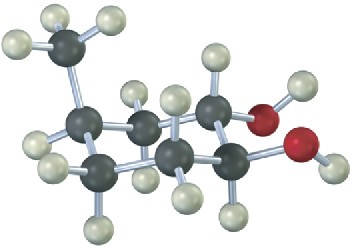
Mechanism Problems
Problem 8-26
Predict the products for the following reactions, showing the complete mechanism and appropriate stereochemistry:
(a)
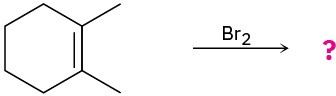
(b)
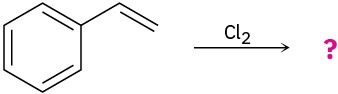
(c)
![]()
Problem 8-27
Draw the structures of the organoboranes formed when borane reacts with the following alkenes, including the regiochemistry and stereochemistry as appropriate. Propose a mechanism for each reaction.
(a)
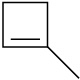
(b)
![]()
(c)
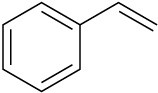
Problem 8-28
meta-Chlorobenzoic acid is not the only peroxyacid capable of epoxide formation. For each reaction below, predict the products and show the mechanism.
(a)
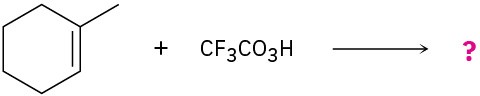
(b)
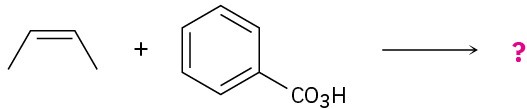
Problem 8-29
Give the mechanism and products for the following acid-catalyzed epoxide-opening reactions, including appropriate stereochemistry.
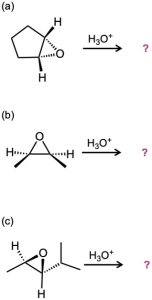
Problem 8-30
Which of the reactions below would result in a product mixture that would rotate plane- polarized light?
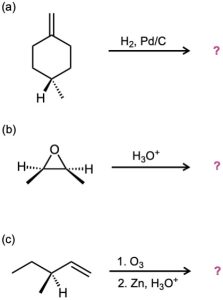
Problem 8-31
Reaction of 2-methylpropene with CH3OH in the presence of H2SO4 catalyst yields methyl tert-butyl ether, CH3OC(CH3)3, by a mechanism analogous to that of acid-catalyzed alkene hydration. Write the mechanism, using curved arrows for each step.
Problem 8-32
Iodine azide, IN3, adds to alkenes by an electrophilic mechanism similar to that of bromine. If a monosubstituted alkene such as 1-butene is used, only one product results:
![]()
(a) Add lone-pair electrons to the structure shown for IN3, and draw a second resonance form for the molecule.
(b) Calculate formal charges for the atoms in both resonance structures you drew for IN3 in part (a).
(c) In light of the result observed when IN3 adds to 1-butene, what is the polarity of the I−N3 bond? Propose a mechanism for the reaction using curved arrows to show the electron flow in each step.
Problem 8-33
10-Bromo-α-chamigrene, a compound isolated from marine algae, is thought to be biosynthesized from γ-bisabolene by the following route:
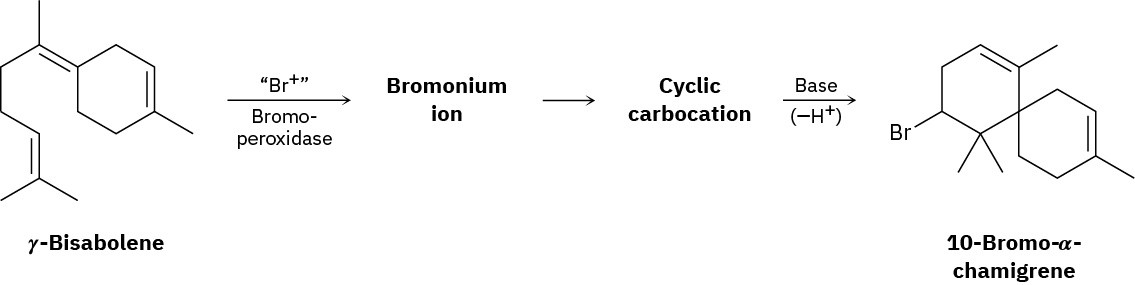
Draw the structures of the intermediate bromonium and cyclic carbocation, and propose mechanisms for all three steps.
Problem 8-34
Isolated from marine algae, prelaureatin is thought to be biosynthesized from laurediol by the following route. Propose a mechanism.
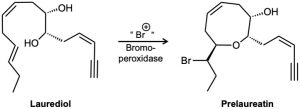
Problem 8-35
Dichlorocarbene can be generated by heating sodium trichloroacetate. Propose a mechanism for the reaction, and use curved arrows to indicate the movement of electrons in each step. What relationship does your mechanism bear to the base-induced elimination of HCl from chloroform?
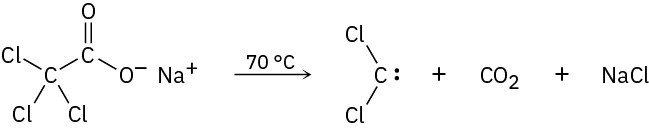
Problem 8-36
Reaction of cyclohexene with mercury(II) acetate in CH3OH rather than H2O, followed by treatment with NaBH4, yields cyclohexyl methyl ether rather than cyclohexanol. Suggest a mechanism.
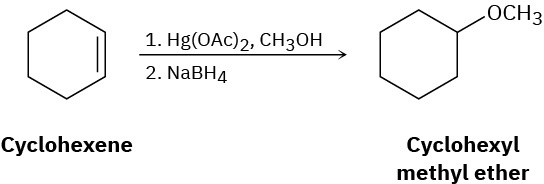
Problem 8-37
Use your general knowledge of alkene chemistry to suggest a mechanism for the following reaction.
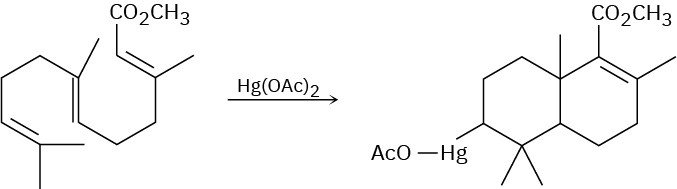
Problem 8-38
Treatment of 4-penten-1-ol with aqueous Br2 yields a cyclic bromo ether rather than the expected bromohydrin. Suggest a mechanism, using curved arrows to show electron movement.
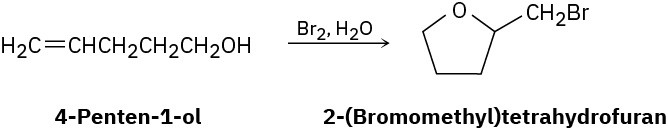
Problem 8-39
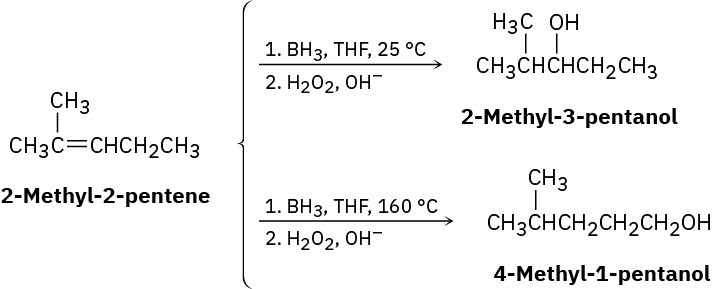
Hydroboration of 2-methyl-2-pentene at 25 °C, followed by oxidation with alkaline H2O2, yields 2-methyl-3-pentanol, but hydroboration at 160 °C followed by oxidation yields 4- methyl-1-pentanol. Suggest a mechanism.
Reactions of Alkenes
Problem 8-40
Predict the products of the following reactions (the aromatic ring is unreactive in all cases). Indicate regiochemistry when relevant.
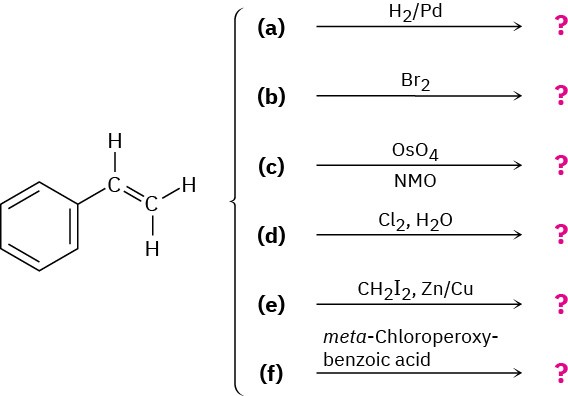
Problem 8-41
Suggest structures for alkenes that give the following reaction products. There may be more than one answer for some cases.
(a)
![]()
(b)
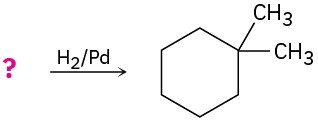
(c)
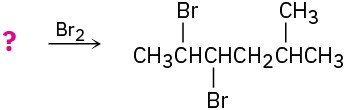
(d)
(e)
![]()
(f)
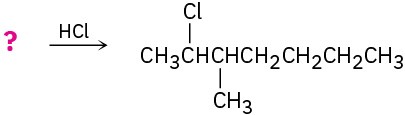
Problem 8-42
Predict the products of the following reactions, showing both regiochemistry and stereochemistry where appropriate:
(a)
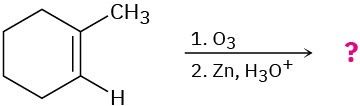
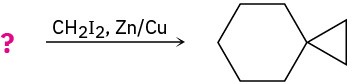
(b)
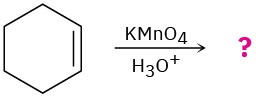
(c)
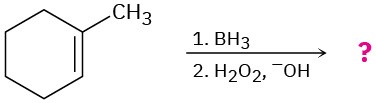
(d)
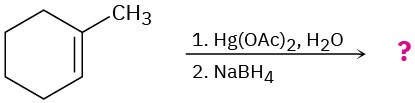
Problem 8-43
Which reaction would you expect to be faster, addition of HBr to cyclohexene or to 1- methylcyclohexene? Explain.
Problem 8-44
What product will result from hydroboration–oxidation of 1-methylcyclopentene with deuterated borane, BD3? Show both the stereochemistry (spatial arrangement) and the regiochemistry (orientation) of the product.
Problem 8-45
The cis and trans isomers of 2-butene give different cyclopropane products in the Simmons–Smith reaction. Show the structures of both, and explain the difference.
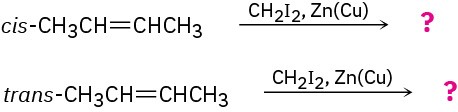
Problem 8-46
Predict the products of the following reactions. Don’t worry about the size of the molecule; concentrate on the functional groups.
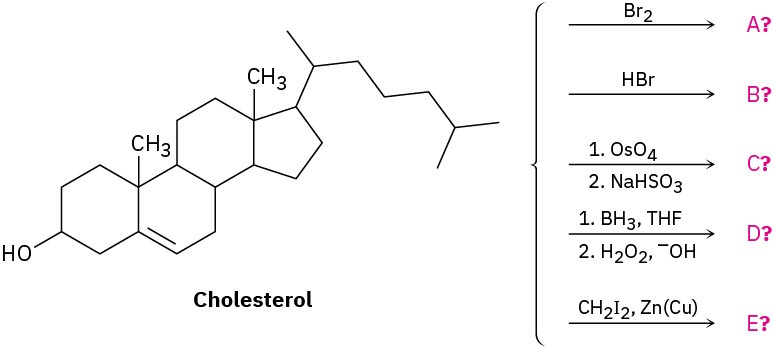
Problem 8-47
Addition of HCl to 1-methoxycyclohexene yields 1-chloro-1-methoxycyclohexane as a sole product. Use resonance structures of the carbocation intermediate to explain why none of the alternate regioisomer is formed.
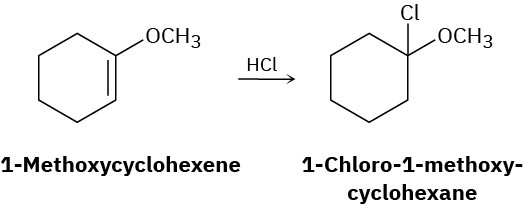
Synthesis Using Alkenes
Problem 8-48
How would you carry out the following transformations? What reagents would you use in each case?
(a)
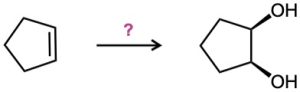
(b)
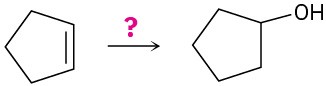
(c)
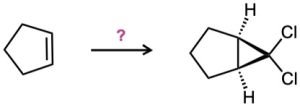
(d)
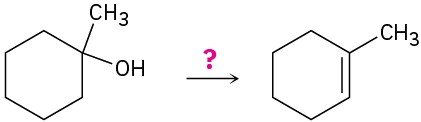
(e)
![]()
(f)
![]()
Problem 8-49
Draw the structure of an alkene that yields only acetone, (CH3)2C=O, on ozonolysis followed by treatment with Zn.
Problem 8-50
Show the structures of alkenes that give the following products on oxidative cleavage with KMnO4 in acidic solution:
(a)
![]()
(b)
![]()
(c)
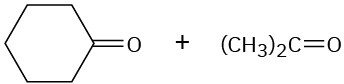
(d)
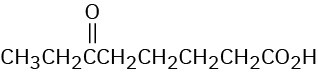
Problem 8-51
In planning the synthesis of one compound from another, it’s just as important to know what not to do as to know what to do. The following reactions all have serious drawbacks to them. Explain the potential problems of each.
(a)
![]()
(b)

(c)
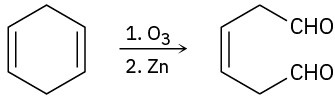
(d)

Problem 8-52
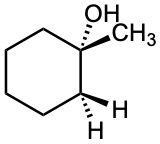
Which of the following alcohols could not be made selectively by hydroboration–oxidation of an alkene? Explain.
(a) 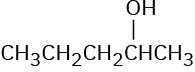
(b) 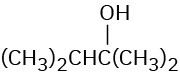
(c)

(d)
Polymers
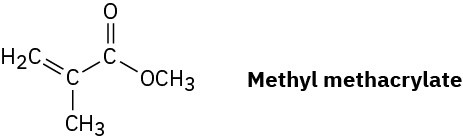
Problem 8-53
Plexiglas, a clear plastic used to make many molded articles, is made by polymerization of methyl methacrylate. Draw a representative segment of Plexiglas.
Problem 8-54
Poly(vinyl pyrrolidone), prepared from N-vinylpyrrolidone, is used both in cosmetics and as a component of a synthetic substitute for blood. Draw a representative segment of the polymer.
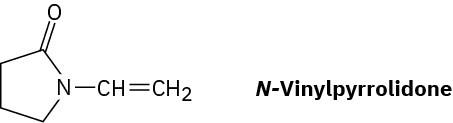
Problem 8-55
When a single alkene monomer, such as ethylene, is polymerized, the product is a homopolymer. If a mixture of two alkene monomers is polymerized, however, a copolymer often results. The following structure represents a segment of a copolymer called Saran. What two monomers were copolymerized to make Saran?
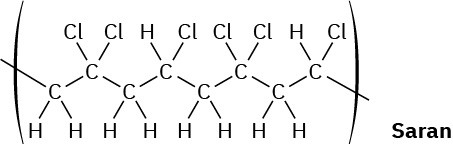
General Problems
Problem 8-56
Compound A has the formula C10H16. On catalytic hydrogenation over palladium, it reacts with only 1 molar equivalent of H2. Compound A also undergoes reaction with ozone, followed by zinc treatment, to yield a symmetrical diketone, B (C10H16O2).
(a) How many rings does A have?
(b) What are the structures of A and B?
(c) Write the reactions.
Problem 8-57
An unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions.
Problem 8-58
Using an oxidative cleavage reaction, explain how you would distinguish between the following two isomeric dienes:
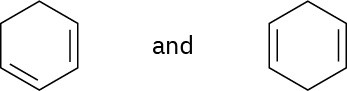
Problem 8-59
Compound A, C10H18O, undergoes reaction with dilute H2SO4 at 50 °C to yield a mixture of two alkenes, C10H16. The major alkene product, B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Identify A and B, and write the reactions.

Problem 8-60
Draw the structure of a hydrocarbon that absorbs 2 molar equivalents of H2 on catalytic hydrogenation and gives only butanedial on ozonolysis.
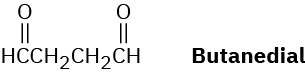
Problem 8-61
Simmons–Smith reaction of cyclohexene with diiodomethane gives a single cyclopropane product, but the analogous reaction of cyclohexene with 1,1-diiodoethane gives (in low yield) a mixture of two isomeric methylcyclopropane products. What are the two products, and how do they differ?
Problem 8-62
The sex attractant of the common housefly is a hydrocarbon with the formula C23H46. On treatment with aqueous acidic KMnO4, two products are obtained, CH3(CH2)12CO2H and CH3(CH2)7CO2H. Propose a structure.
Problem 8-63
Compound A has the formula C8H8. It reacts rapidly with KMnO4 to give CO2 and a carboxylic acid, B (C7H6O2), but reacts with only 1 molar equivalent of H2 on catalytic hydrogenation over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, 4 equivalents of H2 are taken up and hydrocarbon C (C8H16) is produced. What are the structures of A, B, and C? Write the reactions.
Problem 8-64
How would you distinguish between the following pairs of compounds using simple chemical tests? Tell what you would do and what you would see.
(a) cyclopentene and cyclopentane
(b) 2-hexene and benzene
Problem 8-65
α-Terpinene, C10H16, is a pleasant-smelling hydrocarbon that has been isolated from oil of marjoram. On hydrogenation over a palladium catalyst, α-terpinene reacts with 2 molar equivalents of H2 to yield a hydrocarbon, C10H20. On ozonolysis, followed by reduction with zinc and acetic acid, α-terpinene yields two products, glyoxal and 6-methyl-2,5- heptanedione.
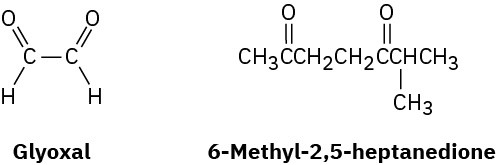
(a) How many degrees of unsaturation does α-terpinene have?
(b) How many double bonds and how many rings does it have?
(c) Propose a structure for α-terpinene.
Problem 8-66
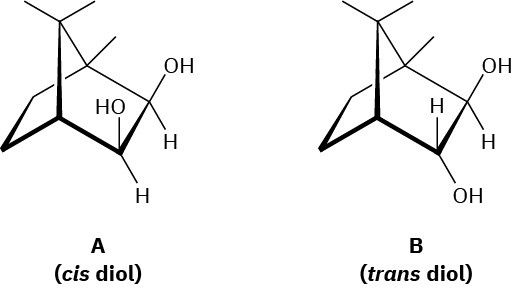
Evidence that cleavage of 1,2-diols by HIO4 occurs through a five-membered cyclic periodate intermediate is based on the measurement of reaction rates. When diols A and B were prepared and the rates of their reaction with HIO4 were measured, it was found that diol A cleaved approximately 1 million times faster than diol B. Make molecular models of A and B and of potential cyclic periodate intermediates, and then explain the results.
Problem 8-67
Reaction of HBr with 3-methylcyclohexene yields a mixture of four products: cis- and trans- 1-bromo-3-methylcyclohexane and cis- and trans-1-bromo-2-methylcyclohexane. The analogous reaction of HBr with 3-bromocyclohexene yields trans-1,2-dibromocyclohexane as the sole product. Draw structures of the possible intermediates, and then explain why only a single product is formed in this reaction.

Problem 8-68
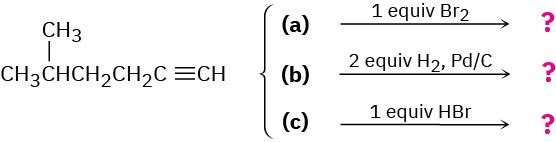
We’ll see in the next chapter that alkynes undergo many of the same reactions that alkenes do. What product might you expect from each of the following reactions?
Problem 8-69
Hydroxylation of cis-2-butene with OsO4 yields a different product than hydroxylation of trans-2-butene. Draw the structure, show the stereochemistry of each product, and explain the difference between them.
Problem 8-70
Compound A, C11H16O, was found to be an optically active alcohol. Despite its apparent unsaturation, no hydrogen was absorbed on catalytic reduction over a palladium catalyst. On treatment of A with dilute sulfuric acid, dehydration occurred and an optically inactive alkene B, C11H14, was the major product. Alkene B, on ozonolysis, gave two products. One product was identified as propanal, CH3CH2CHO. Compound C, the other product, was shown to be a ketone, C8H8O. How many degrees of unsaturation does A have? Write the reactions, and identify A, B, and C.

