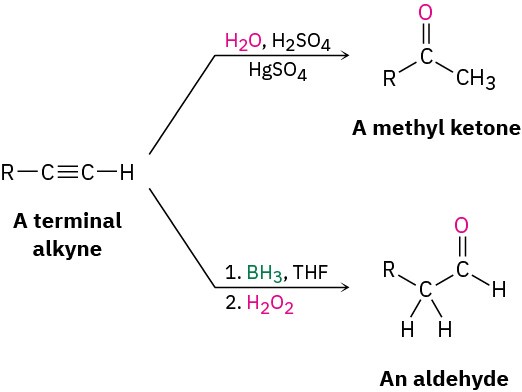
Like alkenes (Section 8.4 and Section 8.5), alkynes can be hydrated by either of two methods. Direct addition of water catalyzed by mercury(II) ion yields the Markovnikov product, and indirect addition of water by a hydroboration–oxidation sequence yields the antiMarkovnikov product.
Mercury(II)-Catalyzed Hydration of Alkynes
Alkynes don’t react directly with aqueous acid but will undergo hydration readily in the presence of mercury(II) sulfate as a Lewis acid catalyst. The reaction occurs with Markovnikov regiochemistry, so the −OH group adds to the more substituted carbon and the −H attaches to the less substituted one.
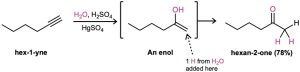
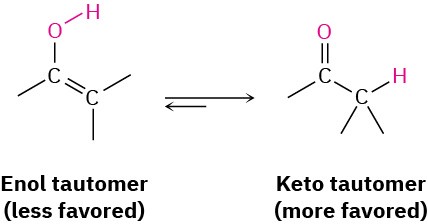
Interestingly, the actual product isolated from alkyne hydration is not a vinylic alcohol, or enol (ene + ol), but is instead a ketone. Although the enol is an intermediate in the reaction, it immediately rearranges into a ketone by a process called keto–enol tautomerism. The individual keto and enol forms are said to be tautomers, a word used to describe two isomers that undergo spontaneous interconversion accompanied by the change in position of a hydrogen. With few exceptions, the keto–enol tautomeric equilibrium lies on the side of the ketone; enols are almost never isolated. We’ll look more closely at this equilibrium in Section 22.1.
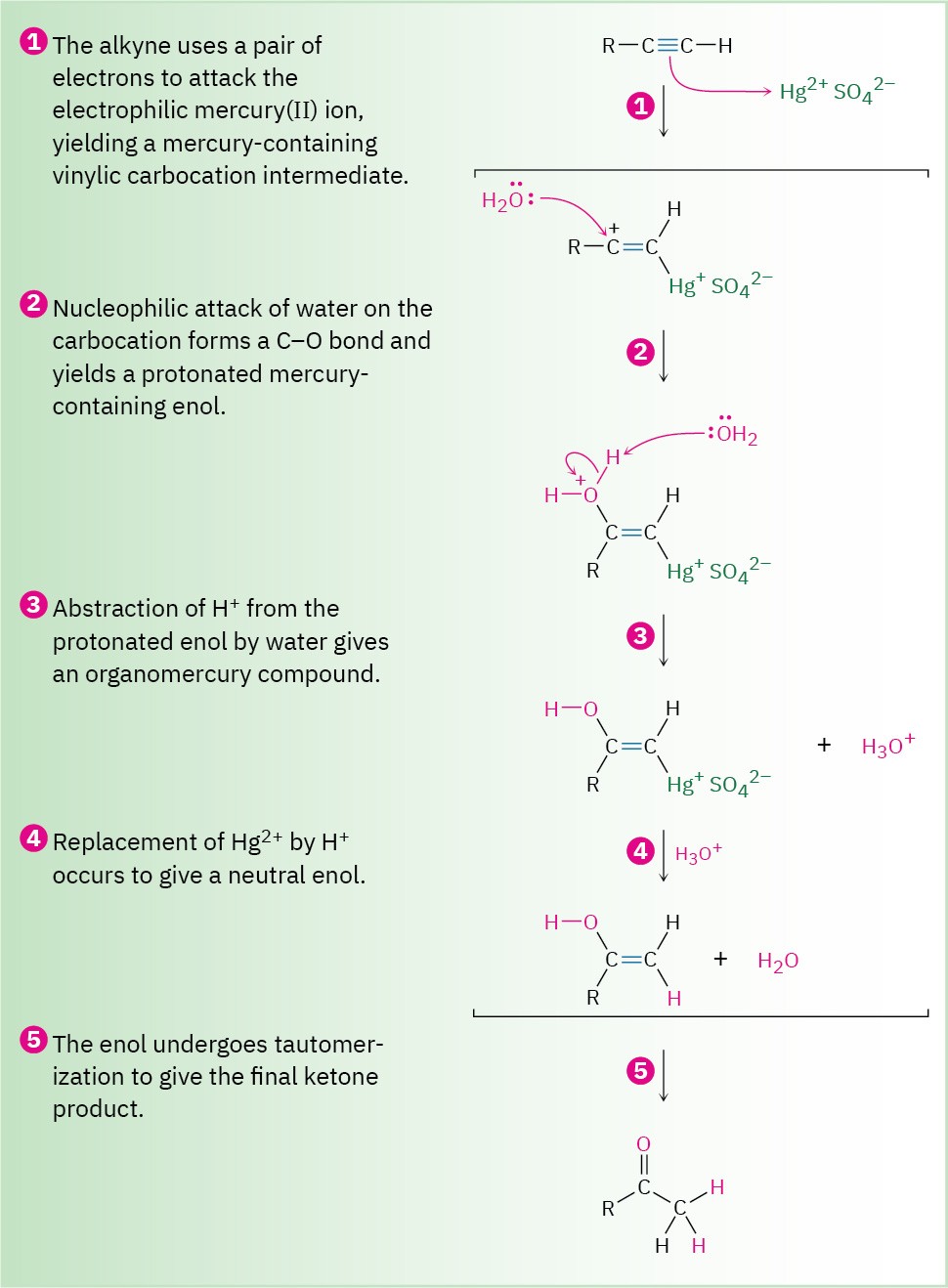
As shown in Figure 9.4, the mechanism of the mercury(II)-catalyzed alkyne hydration reaction is analogous to the oxymercuration reaction of alkenes (Section 8.4). Electrophilic addition of mercury(II) ion to the alkyne gives a vinylic cation, which reacts with water and loses a proton to yield a mercury-containing enol intermediate. In contrast with alkene oxymercuration, however, no treatment with NaBH4 is necessary to remove the mercury.
The acidic reaction conditions alone are sufficient to effect replacement of mercury by hydrogen. Tautomerization then gives the ketone.
Figure 9.4 MECHANISM
Mechanism of the mercury(II)-catalyzed hydration of an alkyne to yield a ketone. The reaction occurs through initial formation of an intermediate enol, which tautomerizes to the ketone.
A mixture of both possible ketones results when an unsymmetrically substituted internal alkyne (RC≡CR’) is hydrated. The reaction is therefore most useful when applied to a terminal alkyne (RC≡CH) because only a methyl ketone is formed.
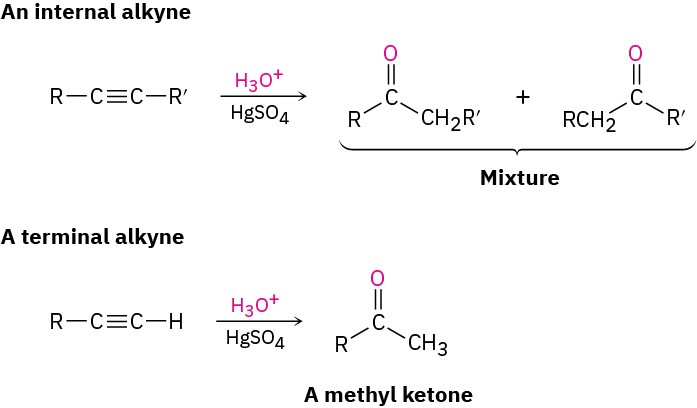
Problem 9-4 What products would you obtain by mercury-catalyzed hydration of the following alkynes?
(a)
![]()
(b)
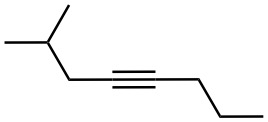
Problem 9-5
What alkynes would you start with to prepare the following ketones?
(a)
![]()
(b)
![]()
Hydroboration–Oxidation of Alkynes
Borane adds rapidly to an alkyne just as it does to an alkene, and the resulting vinylic borane can be oxidized by H2O2 to give an enol, which tautomerizes to either a ketone or an aldehyde, depending on the alkyne. Hydroboration–oxidation of an internal alkyne such as 3-hexyne is straightforward and gives a ketone, but hydroboration–oxidation of a terminal alkyne is more complex because two molecules of borane often add to the triple bond, complicating the situation. To prevent this double addition, a bulky, sterically encumbered borane such as bis(1,2-dimethylpropyl)borane, known commonly as disiamylborane is used in place of BH3. When a terminal alkyne such as 1-butene reacts with disiamylborane, addition to the triple bond occurs normally, but a second addition is hindered by the bulk of the dialkylborane. Oxidation with H2O2 then gives an enol, which tautomerizes to the aldehyde.
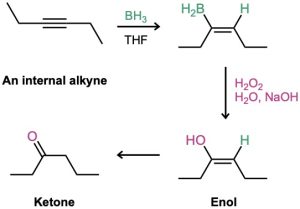
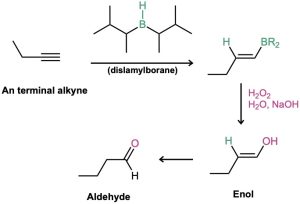
The hydroboration–oxidation sequence is complementary to the direct, mercury(II)- catalyzed hydration reaction of a terminal alkyne because different products result. Direct hydration with aqueous acid and mercury(II) sulfate leads to a methyl ketone, whereas hydroboration–oxidation of the same terminal alkyne leads to an aldehyde.
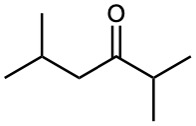
Problem 9-6
What alkyne would you start with to prepare each of the following compounds by a hydroboration–oxidation reaction?
(a)

(b)
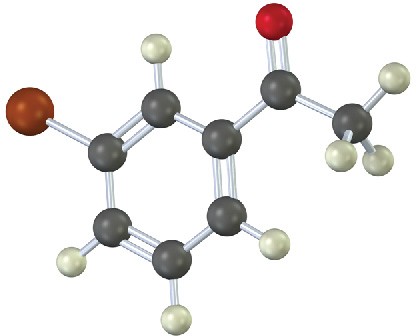
Problem 9-7
How would you prepare the following carbonyl compounds starting from an alkyne (reddish brown = Br)?
(a)
(b)