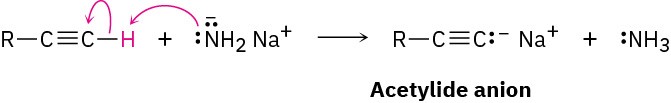
The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. When a terminal alkyne is treated with a strong base, such as sodium amide, NaNH2, the terminal hydrogen is removed and the corresponding acetylide anion is formed.
According to the Brønsted–Lowry definition (Section 2.7), an acid is a substance that donates H+. Although we usually think of oxyacids (H2SO4, HNO3) or halogen acids (HCl, HBr) in this context, any compound containing a hydrogen atom can be an acid under the right circumstances. By measuring dissociation constants of different acids and expressing the results as pKa values, an acidity order can be established. Recall from Section 2.8 that a lower pKa corresponds to a stronger acid and a higher pKa corresponds to a weaker one.
Where do hydrocarbons lie on the acidity scale? As the data in Table 9.1 show, both methane (pKa ≈ 60) and ethylene (pKa = 44) are very weak acids and thus do not react with any of the common bases. Acetylene, however, has pKa = 25 and can be deprotonated by the conjugate base of any acid whose pKa is greater than 25. Amide ion (NH2−), for example, the conjugate base of ammonia (pKa = 35), is often used to deprotonate terminal alkynes.
Table 9.1 Acidity of Simple Hydrocarbons

Why are terminal alkynes more acidic than alkenes or alkanes? In other words, why are acetylide anions more stable than vinylic or alkyl anions? The simplest explanation involves the hybridization of the negatively charged carbon atom. An acetylide anion has an sp-hybridized carbon, so the negative charge resides in an orbital that has 50% “s character.” A vinylic anion has a sp2-hybridized carbon with 33% s character, and an alkyl anion (sp3) has only 25% s character. Because s orbitals are nearer the positive nucleus and lower in energy than p orbitals, the negative charge is stabilized to a greater extent in an orbital with higher s character (Figure 9.6).
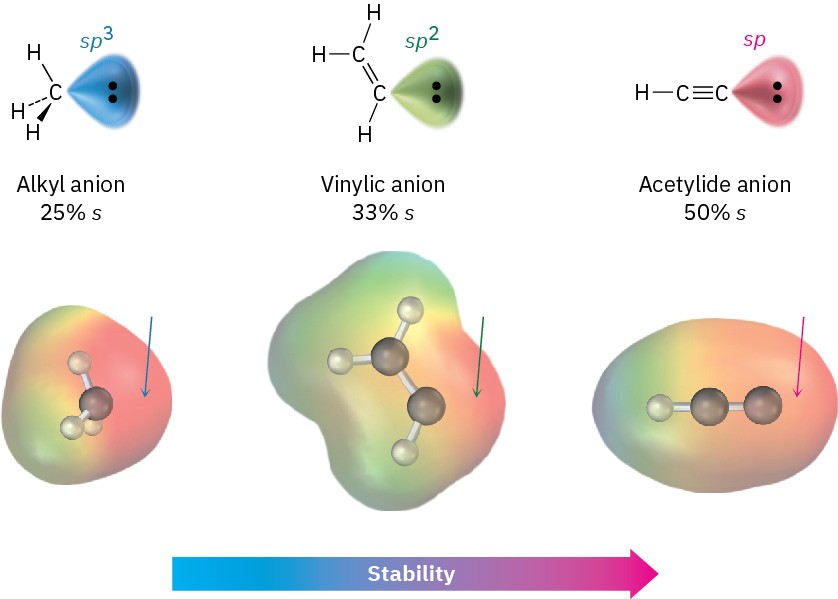
Figure 9.6 A comparison of alkyl, vinylic, and acetylide anions. The acetylide anion, with sp hybridization, has more s character and is more stable. Electrostatic potential maps show that placing the negative charge closer to the carbon nucleus makes carbon appear less negative (red).
Problem 9-9
The pKa of acetone, CH3COCH3, is 19.3. Which of the following bases is strong enough to deprotonate acetone?
(a) KOH (pKa of H2O = 15.7)
(b) NaC≡CH (pKa of C2H2 = 25)
(c) NaHCO3 (pKa of H2CO3 = 6.4)
(d) NaOCH3 (pKa of CH3OH = 15.6)

