When first dealing with resonance forms, it’s useful to have a set of guidelines that describe how to draw and interpret them. The following should be helpful:
Individual resonance forms are imaginary, not real. Molecules do not rapidly convert back and forth between resonance forms. Rather, the “real” structure and properties of the species is an average, or resonance hybrid, of the different depictions provided by the resonance structures.
Resonance forms differ only in the placement of their π and/or nonbonding electrons. Resonance structures are describing the same species. Resonance structure must possess the same number of electrons and the same atom connectivity. This means that when drawing resonance structures, atoms are never “rearranged” and sigma bonds are never broken. Is is simply the π electrons and nonbonding electrons that are “rearranged.” This movement of electrons from one resonance structure to another can be indicated with curved arrows. A curved arrow always indicates the movement of electrons, not the movement of atoms. An arrow shows that a pair of electrons moves from the atom or bond at the tail of the arrow to the atom or bond at the head of the arrow.
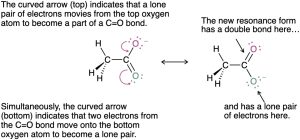
The situation with benzene is similar to that with acetate. The π electrons in the double bonds move, as shown with curved arrows, but the carbon and hydrogen atoms remain in place.
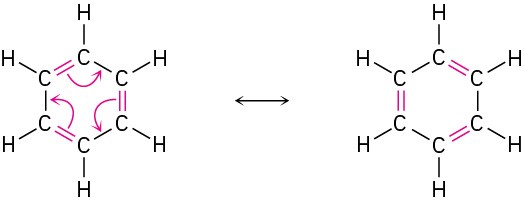
Different resonance forms of a substance don’t have to be equivalent. As an example, we’ll see in Chapter 22 that a compound such as acetone, which contains a C=O bond, can be converted into its anion by reaction with a strong base. The resultant anion has two resonance forms. One form contains a carbon–oxygen double bond and has a negative charge on a carbon; the other contains a carbon–carbon double bond and has a negative charge on oxygen. Even though the two resonance forms aren’t equivalent, both contribute to the overall resonance hybrid.
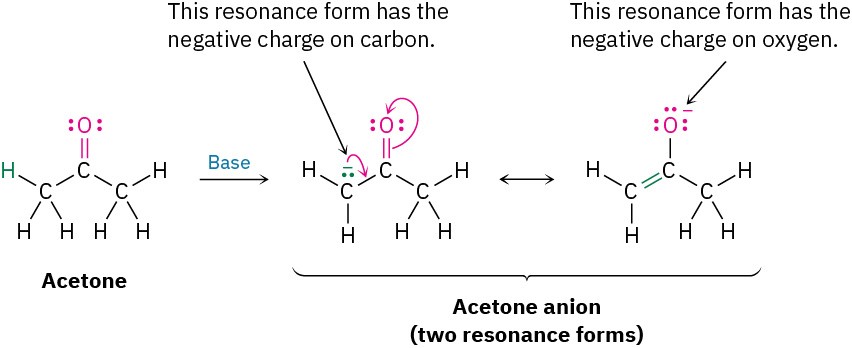
When two resonance forms are nonequivalent, the actual structure of the resonance hybrid resembles the more stable form. Thus, we might expect the true structure of the acetone anion to be more like that of the form that places the negative charge on the electronegative oxygen atom rather than on the carbon.
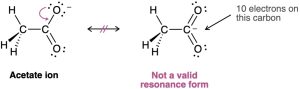
Resonance forms cannot exceed standard valency. When drawing resonance structures, the octet rule is still relevant for second-row, main-group atoms. Consider the following structures for the acetate ion. The structure on the right is not a valid resonance form because the carbon atom has five bonds and has exceeded its octet.
Resonance leads to stability. Generally speaking, the larger the number of resonance forms a substance has, the more stable the substance is. This is because its electrons are spread out over a larger part of the molecule and are attracted to more nuclei.

