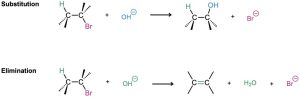
We said at the beginning of this chapter that two kinds of reactions can take place when a nucleophile/Lewis base reacts with an alkyl halide. The nucleophile can either substitute for the halide by reaction at carbon or can cause elimination of HX by reaction at a neighboring hydrogen:
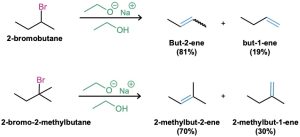
Elimination reactions are more complex than substitution reactions for several reasons. One is the problem of regiochemistry. What product results by loss of HX from an unsymmetrical halide? In fact, elimination reactions almost always give mixtures of alkene products, and the best we can usually do is to predict which will be the major product.
According to Zaitsev’s rule, formulated in 1875 by the Russian chemist Alexander Zaitsev, base-induced elimination reactions generally (although not always) give the more stable alkene product—that is, the alkene with more alkyl substituents on the double-bond carbons. In the following two cases, for example, the more highly substituted alkene product predominates.
ZAITSEV’S RULE
In the elimination of HX from an alkyl halide, the more highly substituted alkene product predominates.
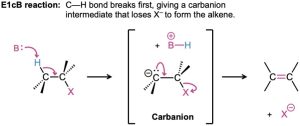
Another factor that complicates a study of elimination reactions is that they can take place by different mechanisms, just as substitutions can. We’ll consider three of the most common mechanisms—the E1, E2, and E1cB reactions—which differ in the timing of C–H and C–X bond-breaking.
In the E1 reaction, the C–X bond breaks first to give a carbocation intermediate, which undergoes subsequent base abstraction of H+ to yield the alkene. In the E2 reaction, base-induced C–H bond cleavage is simultaneous with C–X bond cleavage, giving the alkene in a single step. In the E1cB reaction (cB for “conjugate base”), base abstraction of the proton occurs first, giving a carbanion (R:–) intermediate. This anion, the conjugate base of the reactant “acid,” then undergoes loss of X– in a subsequent step to give the alkene. All three mechanisms occur frequently in the laboratory, but the E1cB mechanism predominates in biological pathways.


Worked Example 11.3 Predicting the Product of an Elimination Reaction
What product would you expect from reaction of 1-chloro-1-methylcyclohexane with KOH in ethanol?
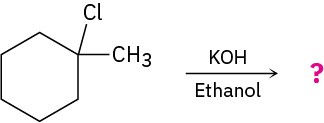
Strategy
Treatment of an alkyl halide with a strong base such as KOH yields an alkene. To find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then generate the potential alkene products by removing HX in as many ways as possible. The major product will be the one that has the most highly substituted double bond—in this case, 1-methylcyclohexene.
Solution
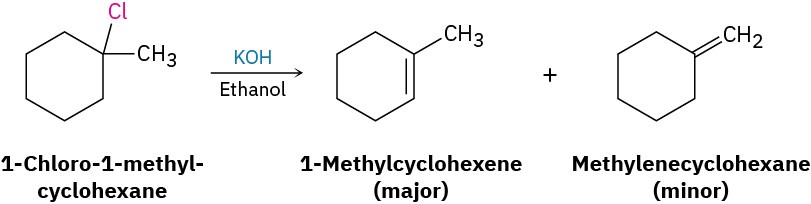
Problem 11-15
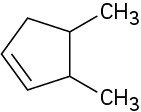
Ignoring double-bond stereochemistry, what products would you expect from elimination reactions of the following alkyl halides? Which product will be the major product in each case?
(a)
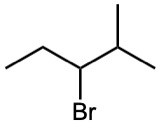
(b)
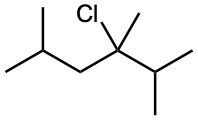
(c)
![]()
Problem 11-16
What alkyl halides might the following alkenes have been made from?
(a)

(b)