In infrared (IR) spectroscopy, the IR region of the electromagnetic spectrum covers the range from just above the visible (7.8 × 10–7 m) to approximately 10–4 m, but only the midportion from 2.5 × 10–6 m to 2.5 × 10–5 m is used by organic chemists (Figure 12.20). Wavelengths within the IR region are usually given in micrometers (1 μm = 10-6 m), and frequencies are given in wavenumbers rather than in hertz. The wavenumber![]() is the reciprocal of wavelength in centimeters and is therefore expressed in units of cm–1.
is the reciprocal of wavelength in centimeters and is therefore expressed in units of cm–1.
Wavenumber: ![]() (cm-1) = 1 / 𝜆 (cm)
(cm-1) = 1 / 𝜆 (cm)
Thus, the useful IR region is from 4000 to 400 cm–1, corresponding to energies of 48.0 kJ/mol to 4.80 kJ/mol (11.5–1.15 kcal/mol).
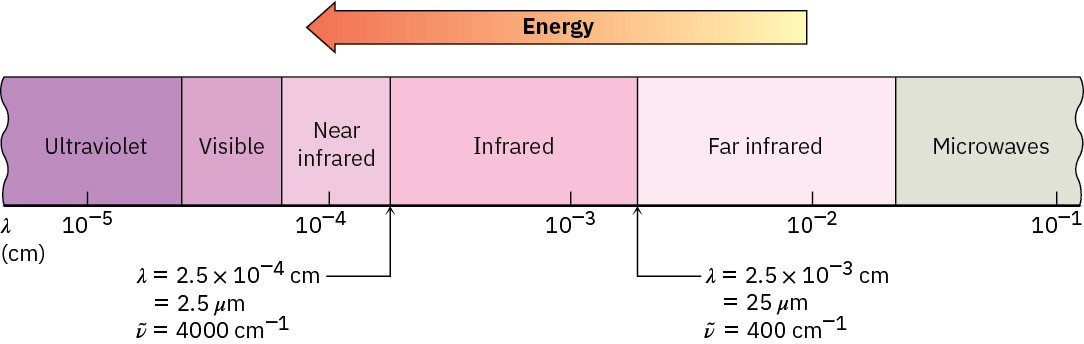
Figure 12.20 The infrared and adjacent regions of the electromagnetic spectrum.
Why does an organic molecule absorb some wavelengths of IR radiation but not others? All molecules have a certain amount of energy and are in constant motion. Their bonds stretch and contract, atoms wag back and forth, and other molecular vibrations occur. Some of the kinds of allowed vibrations are shown below:
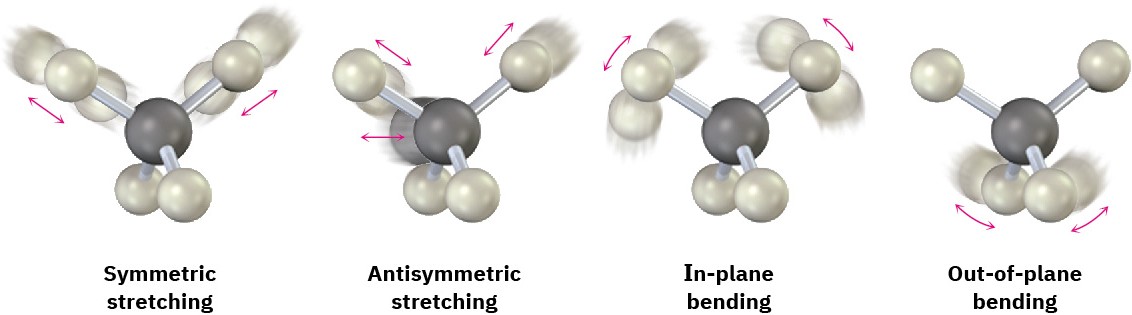
The amount of energy a molecule contains is not continuously variable but is quantized. That is, a molecule can stretch or bend only at specific frequencies corresponding to specific energy levels. Take bond stretching, for example. Although we usually speak of bond lengths as if they were fixed, the numbers given are really averages. In fact, a typical C–H bond with an average bond length of 110 pm is actually vibrating at a specific frequency, alternately stretching and contracting as if there were a spring connecting the two atoms.
When a molecule is irradiated with electromagnetic radiation, energy is absorbed if the frequency of the radiation matches the frequency of the vibration. The result of this energy absorption is an increased amplitude for the vibration; in other words, the “spring” connecting the two atoms stretches and compresses a bit further. Since each frequency absorbed by a molecule corresponds to a specific molecular motion, we can find what kinds of motions a molecule has by measuring its IR spectrum. By interpreting these motions, we can find out what kinds of bonds (functional groups) are present in the molecule.
IR spectrum → What molecular motions? → What functional groups?

