The information derived from 13C NMR spectroscopy is extraordinarily useful for structure determination. Not only can we count the number of nonequivalent carbon atoms in a molecule, we can also get information about the electronic environment of each carbon and find how many protons are attached to each. As a result, we can address many structural questions that go unanswered by IR spectroscopy or mass spectrometry.
Here’s an example: how do we know that the E2 reaction of an alkyl halide follows Zaitsev’s rule (Section 11.7)? Does treatment of 1-chloro-1-methylcyclohexane with a strong base give predominantly the trisubstituted alkene 1-methylcyclohexene or the disubstituted alkene methylenecyclohexane?
1-Methylcyclohexene will have five sp3-carbon resonances in the 20 to 50 δ range and two sp2-carbon resonances in the 100 to 150 δ range. Methylenecyclohexane, however, because of its symmetry, will have only three sp3-carbon resonance peaks and two sp2-carbon peaks. The spectrum of the actual reaction product, shown in Figure 13.22, clearly identifies 1-methylcyclohexene as the product of this E2 reaction.
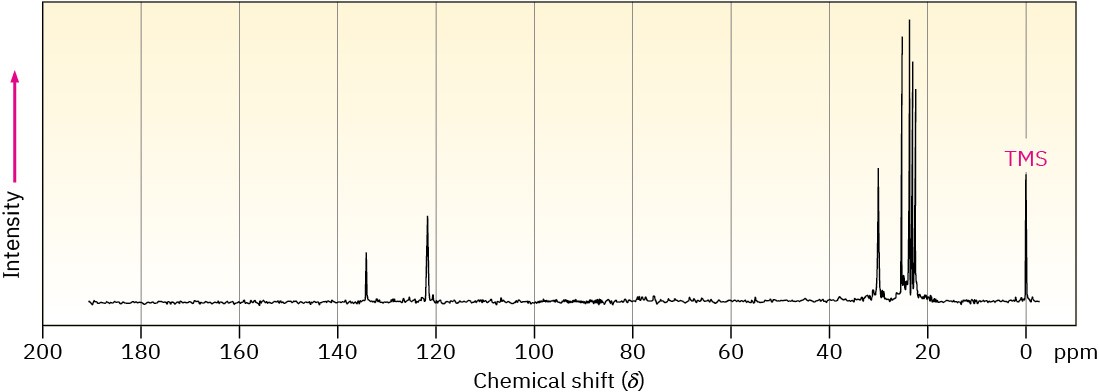
Figure 13.22 The 13C NMR spectrum of 1-methylcyclohexene, the E2 reaction product from treatment of 1-chloro-1-methylcyclohexane with base.
Problem 13-23
We saw in Section 9.3 that addition of HBr to a terminal alkyne leads to the Markovnikov addition product, with the Br bonding to the more highly substituted carbon. How could you use 13C NMR to identify the product of the addition of 1 equivalent of HBr to 1-hexyne?

