As we’ve seen, mass spectrometry, infrared spectroscopy, and nuclear magnetic resonance spectroscopy are techniques of structure determination applicable to all organic molecules. In addition to these three generally useful methods, there is a fourth—ultraviolet (UV) spectroscopy—that is applicable only to conjugated compounds. UV is less commonly used than the other three spectroscopic techniques because of the specialized information it gives, so we’ll only discuss it briefly.
|
Mass spectrometry |
Molecular size and formula |
|
IR spectroscopy |
Functional groups present |
|
NMR spectroscopy |
Carbon–hydrogen framework |
|
UV spectroscopy |
Conjugated π electron systems |
The ultraviolet region of the electromagnetic spectrum extends from the short-wavelength end of the visible region (4 × 10–7 m) to the long-wavelength end of the X-ray region (10–8 m), but the narrow range from 2 × 10–7 m to 4 × 10–7 m is the part of greatest interest to organic chemists. Absorptions in this region are usually measured in nanometers (nm), where 1 nm = 10–9 m. Thus, the ultraviolet range of interest is from 200 to 400 nm (Figure 14.11).
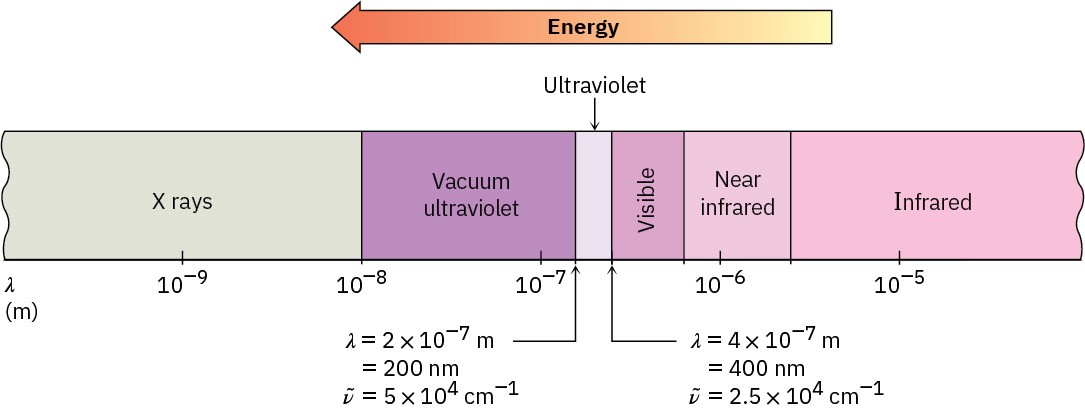
Figure 14.11The ultraviolet (UV) and neighboring regions of the electromagnetic spectrum.
We saw in Section 12.5 that when an organic molecule is irradiated with electromagnetic energy, the radiation either passes through the sample or is absorbed, depending on its energy. With IR irradiation, the energy absorbed corresponds to the amount needed to increase molecular vibrations. With UV radiation, the energy absorbed corresponds to the amount needed to promote an electron from a lower-energy orbital to a higher-energy one in a conjugated molecule. The conjugated diene 1,3-butadiene, for instance, has four π molecular orbitals, as shown previously in Figure 14.3. The two lower-energy, bonding MOs are occupied in the ground state, and the two higher-energy, antibonding MOs are unoccupied. On irradiation with ultraviolet light (hυ), 1,3-butadiene absorbs energy and a π electron is promoted from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). Because the electron is promoted from a bonding π molecular orbital to an antibonding π* molecular orbital, we call this a π → π* excitation (read as “pi to pi star”). The energy gap between the HOMO and the LUMO of 1,3-butadiene is such that UV light of 217 nm wavelength is required to effect the π → π* electronic transition (Figure 14.12).
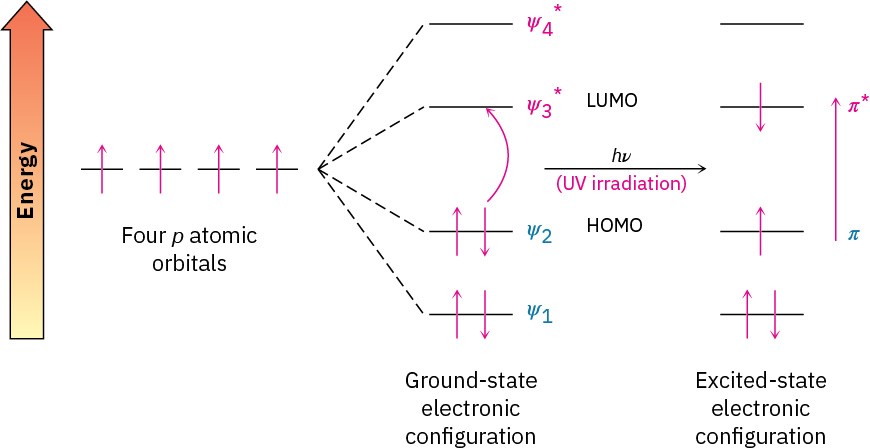
Figure 14.12Ultraviolet excitation of 1,3-butadiene results in the promotion of an electron from ψ2, the highest occupied molecular orbital (HOMO), to ψ3*, the lowest unoccupied molecular orbital (LUMO).
An ultraviolet spectrum is recorded by irradiating a sample with UV light of continuously changing wavelength. When the wavelength corresponds to the energy level required to excite an electron to a higher level, energy is absorbed. This absorption is detected and displayed on a chart that plots wavelength versus absorbance (A), defined as
![]()
where I0 is the intensity of the incident light and I is the intensity of the light transmitted through the sample.
Note that UV spectra differ from IR spectra in how they are presented. For historical reasons, IR spectra are usually displayed so that the baseline corresponding to zero absorption runs across the top of the chart and a valley indicates an absorption, whereas UV spectra are displayed with the baseline at the bottom of the chart so that a peak indicates an absorption (Figure 14.13).
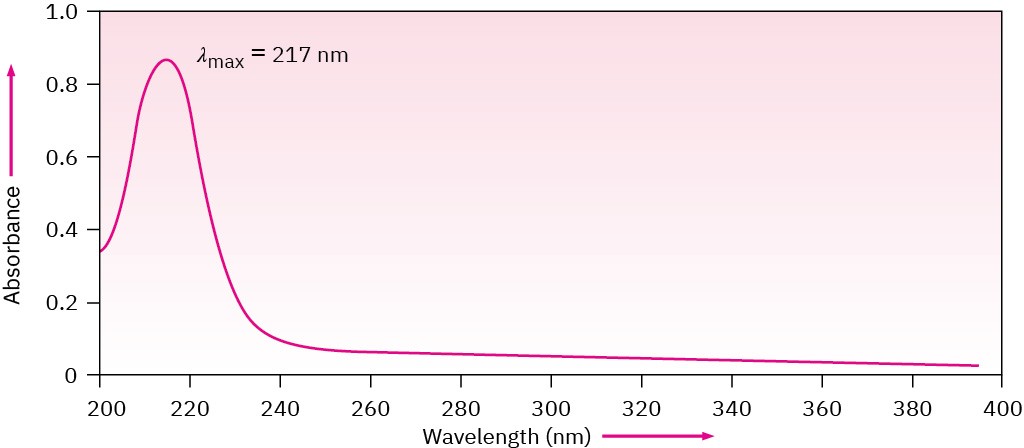
Figure 14.13The ultraviolet spectrum of 1,3-butadiene, λmax = 217 nm.
The amount of UV light absorbed is expressed as the sample’s molar absorptivity (ϵ), defined by the equation where
![]()

Molar absorptivity is a physical constant, characteristic of the particular substance being observed and thus characteristic of the particular π electron system in the molecule. Typical values for conjugated dienes are in the range ε = 10,000 to 25,000. The units for molar absorptivity, L/(mol · cm), are usually dropped. A particularly important use of this equation comes from rearranging it to the form [latex]c=\frac{A}{𝜀 × l}[/latex], which lets us measure the concentration of a sample in solution when A, ε, and l are known. As an example, β-carotene, the pigment responsible for the orange color of carrots, has ε = 138,000 L/(mol · cm). If a sample of β-carotene is placed in a cell with a pathlength of 1.0 cm and the UV absorbance reads 0.37, then the concentration of β-carotene in the sample is
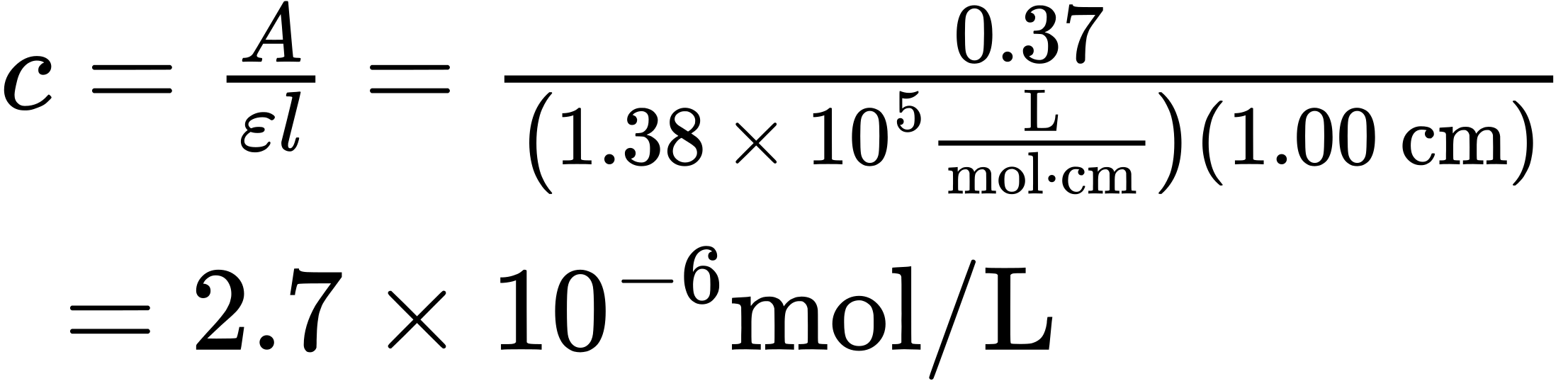
Unlike IR and NMR spectra, which show many absorptions for a given molecule, UV spectra are usually quite simple—often only a single peak. The peak is usually broad, and we identify its position by noting the wavelength at the top of the peak—λmax, read as “lambda max.”
Problem 14-13
Calculate the energy range of electromagnetic radiation in the UV region of the spectrum from 200 to 400 nm (see Section 12.5). How does this value compare with the values calculated previously for IR and NMR spectroscopy?
Problem 14-14
If pure vitamin A has λmax = 325 (ε = 50,100), what is the vitamin A concentration in a sample whose absorbance at 325 nm is A = 0.735 in a cell with a pathlength of 1.00 cm?

