Aldehydes and ketones react with water to yield 1,1-diols, or geminal (gem) diols. The hydration reaction is reversible, and a gem diol can eliminate water to regenerate an aldehyde or ketone.
 The position of the equilibrium between a gem diol and an aldehyde or ketone depends on the structure of the carbonyl compound. Equilibrium generally favors the carbonyl compound for steric reasons, but the gem diol is favored for a few simple aldehydes. For example, an aqueous solution of formaldehyde consists of 99.9% gem diol and 0.1% aldehyde at equilibrium, whereas an aqueous solution of acetone consists of only about 0.1% gem diol and 99.9% ketone.
The position of the equilibrium between a gem diol and an aldehyde or ketone depends on the structure of the carbonyl compound. Equilibrium generally favors the carbonyl compound for steric reasons, but the gem diol is favored for a few simple aldehydes. For example, an aqueous solution of formaldehyde consists of 99.9% gem diol and 0.1% aldehyde at equilibrium, whereas an aqueous solution of acetone consists of only about 0.1% gem diol and 99.9% ketone.
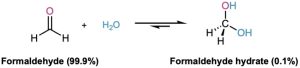
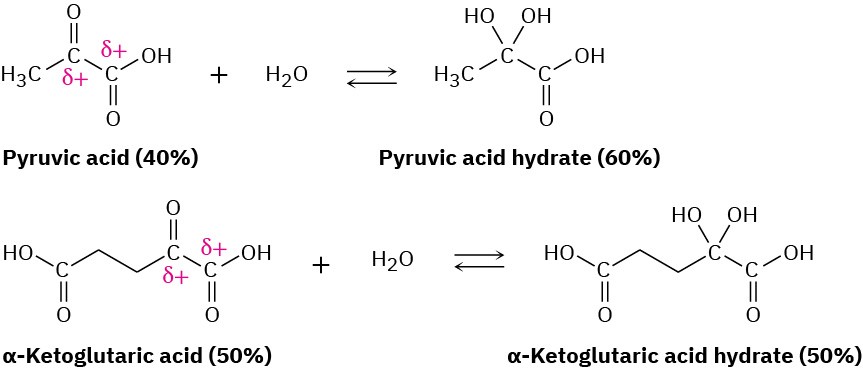
The hydrate is also favored for α-keto carboxylic acids, which have adjacent positively charged carbons that destabilize the keto form and favor the hydrate. Such compounds occur commonly in many biological pathways. Pyruvic acid, which is 60% hydrate at equilibrium, and α-ketoglutaric acid, which is 50% hydrate, are examples.
The nucleophilic addition of water to an aldehyde or ketone is slow under neutral conditions but is catalyzed by both base and acid. Under basic conditions (Figure 19.5a), the nucleophile is negatively charged (OH–) and uses a pair of its electrons to form a bond to the electrophilic carbon atom of the C═O group. At the same time, the C═O carbon atom rehybridizes from sp2 to sp3 and two electrons from the C═O π bond are pushed onto the oxygen atom, giving an alkoxide ion. Protonation of the alkoxide ion by water then yields a neutral addition product plus regenerated OH–.
Under acidic conditions (Figure 19.5b), the carbonyl oxygen atom is first protonated by H3O+ to make the carbonyl group more strongly electrophilic. A neutral nucleophile, H2O, then uses a pair of electrons to bond to the carbon atom of the C═O group, and two electrons from the C═O π bond move onto the oxygen atom. The positive charge on oxygen is thereby neutralized, while the nucleophile gains a positive charge. Finally, deprotonation by water gives the neutral addition product and regenerates the H3O+ catalyst.
Note the key difference between the base-catalyzed and acid-catalyzed reactions. The base- catalyzed reaction takes place rapidly because water is converted into hydroxide ion, a much better nucleophile. The acid-catalyzed reaction takes place rapidly because the carbonyl compound is converted by protonation into a much better electrophile.
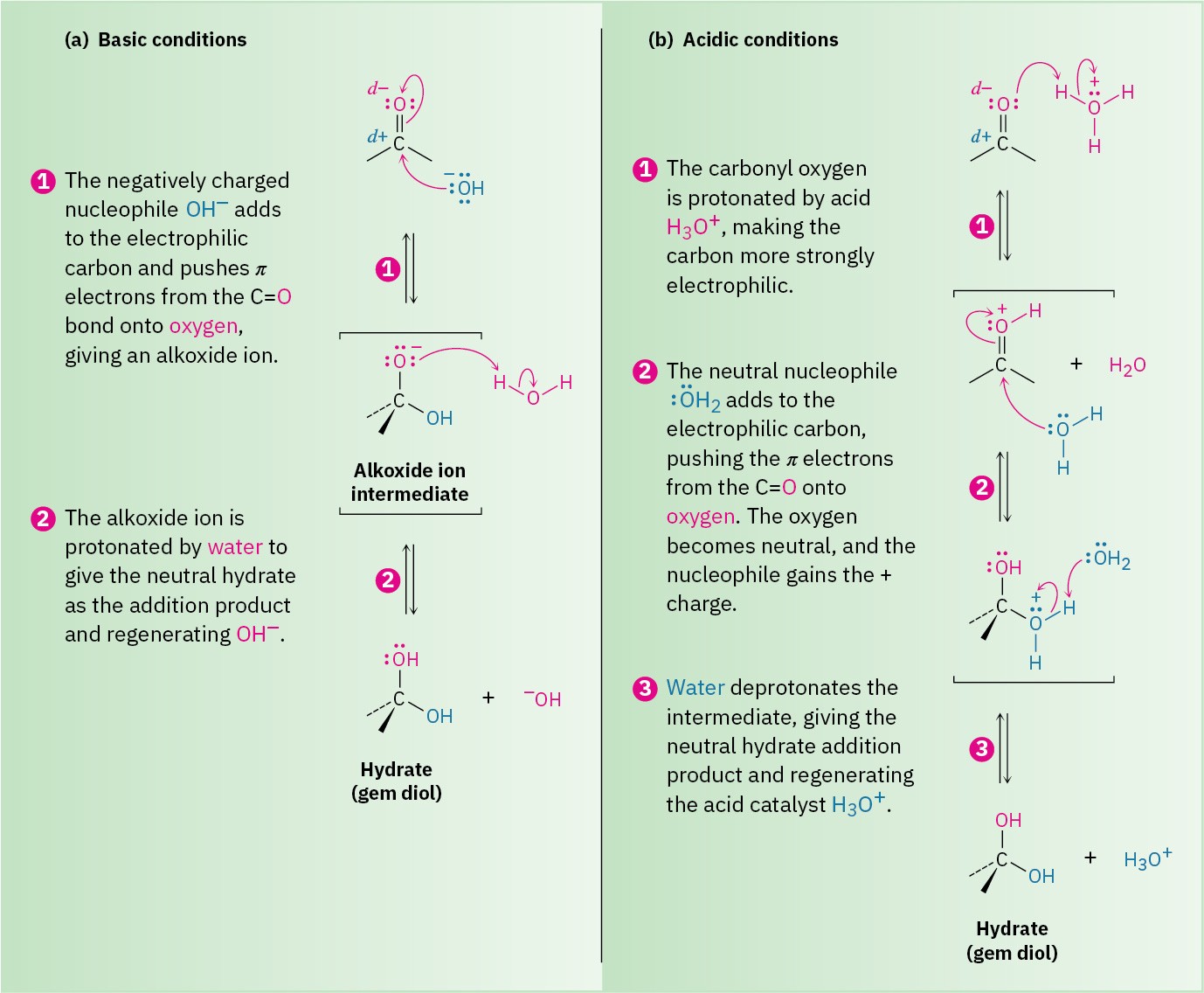
Figure 19.5 The mechanism for a nucleophilic addition reaction of aldehydes and ketones under both basic and acidic conditions. (a) Under basic conditions, a negatively charged nucleophile adds to the carbonyl group to give an alkoxide ion intermediate, which is subsequently protonated. (b) Under acidic conditions, protonation of the carbonyl group occurs first, followed by addition of a neutral nucleophile and subsequent deprotonation.
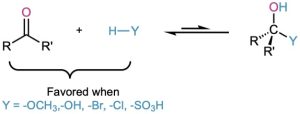
The hydration reaction just described is typical of what happens when an aldehyde or ketone is treated with a nucleophile of the type H–Y, where the Y atom is electronegative and can stabilize a negative charge (oxygen, halogen, or sulfur, for instance). In such reactions, the nucleophilic addition is reversible, with the equilibrium generally favoring the carbonyl reactant rather than the tetrahedral addition product. In other words, treatment of an aldehyde or ketone with CH3OH, H2O, HCl, HBr, or H2SO4 does not normally lead to a stable alcohol addition product.
Problem 19-7
When dissolved in water, trichloroacetaldehyde exists primarily as its hydrate, called chloral hydrate. Show the structure of chloral hydrate.
Problem 19-8
The oxygen in water is primarily (99.8%) 16O, but water enriched with the heavy isotope 18O is also available. When an aldehyde or ketone is dissolved in 18O-enriched water, the isotopic label becomes incorporated into the carbonyl group. Explain.
R2C=O + H2O ⇌ R2C=O + H2O where O = 18O

