By the mid-1800s, the new science of chemistry was developing rapidly, especially in Europe, and chemists had begun to probe the forces holding compounds together. In 1858, the German chemist August Kekulé and the Scottish chemist Archibald Couper independently proposed that, in all organic compounds, carbon is tetravalent—it always forms four bonds when it joins other elements to form stable compounds. Furthermore, said Kekulé, carbon atoms can bond to one another to form extended chains of linked atoms. In 1865, Kekulé provided another major advance when he suggested that carbon chains can double back on themselves to form rings of atoms.
Although Kekulé and Couper were correct in describing the tetravalent nature of carbon, chemistry was still viewed in a two-dimensional way until 1874. In that year, the Dutch chemist Jacobus Van’t Hoff and French chemist Joseph Le Bel added a third dimension to our ideas about organic compounds when they proposed that the four bonds of carbon are not oriented randomly but have specific spatial directions. Van’t Hoff went even further and suggested that the four atoms to which carbon is bonded could sit at the corners of a regular tetrahedron, with carbon in the center.
A representation of this tetrahedral carbon atom is shown in Figure 1.7. Note the conventions used to show three-dimensionality: solid lines represent bonds in the plane of the page, the heavy wedged line represents a bond coming out of the page toward the viewer, and the dashed line represents a bond receding back behind the page, away from the viewer. Get used to them; these representations will be used throughout the text.
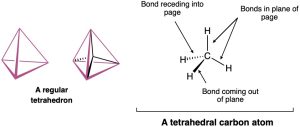
Figure 1.7 A representation of Van’t Hoff’s tetrahedral carbon atom. The solid lines represent bonds in the plane of the paper, the heavy wedged line represents a bond coming out of the plane of the page toward the viewer, and the dashed line represents a bond going back behind the plane of the page away from the viewer.
Why, though, do atoms bond together, and how can chemical bonds be described electronically? The why question is relatively easy to answer: atoms bond together because the compound that results is more stable and lower in energy than the separate atoms.
Energy—usually as heat—is always released and flows out of the chemical system when a bond forms. Conversely, energy is added to the chemical system when a bond breaks. Making bonds always releases energy, and breaking bonds always absorbs energy. The how question is more difficult. To answer it, we need to know more about the electronic properties of atoms.
We know through observation that eight electrons (octet) in an atom’s outermost shell, or valence shell, impart special stability to the noble-gas elements in group 8A of the periodic table: Ne (2 + 8); Ar (2 + 8 + 8); Kr (2 + 8 + 18 + 8). We also know that the chemistry of the main-group elements on the left and right sides of the periodic table is governed by their tendency to take on the electron configuration of the nearest noble gas.
The alkali metals such as sodium in group 1A, for example, achieve a noble-gas configuration by losing the single s electron from their valence shell to form a cation, while the halogens such as chlorine in group 7A achieve a noble-gas configuration by gaining a p electron to fill their valence shell and form an anion. The resultant ions are held together in compounds like Na+ Cl– by the electrical attraction of unlike charges that we call an ionic bond.
But how do elements closer to the middle of the periodic table form bonds? Look at methane, CH4, the main constituent of natural gas, for example. The bonding in methane is not ionic because it would take too much energy for carbon (1s2 2s2 2p2) either to gain or lose four electrons to achieve a noble-gas configuration. Instead, carbon bonds to other atoms, not by gaining or losing electrons, but by sharing them. Such a shared-electron bond, first proposed in 1916 by the American chemist G. N. Lewis, is called a covalent bond. The neutral collection of atoms held together by covalent bonds is called a molecule. Ionic compounds such as sodium chloride, however, are not called molecules.
A simple way of indicating the covalent bonds in molecules is to use what are called Lewis structures, in which the valence-shell electrons of an atom are represented as either dots or lines (a line is representative of two electrons being shared between atoms in a covalent bond). A stable molecule results whenever a noble-gas configuration of eight valence electrons (an octet) is achieved for all main-group atoms or two dots for hydrogen.
The number of covalent bonds an atom forms depends on how many additional valence electrons it needs to reach a noble-gas configuration. Hydrogen has one valence electron (1s) and needs only one more to reach the helium configuration (1s2), so it forms one bond. Carbon has four valence electrons (2s2 2p2) and needs four more to reach the neon configuration (2s2 2p6), so it forms four bonds. Nitrogen has five valence electrons (2s2 2p3), needs three more, and forms three bonds. Oxygen has six valence electrons (2s2 2p4), needs two more, and forms two bonds. The halogens have seven valence electrons, need one more, and form one bond.
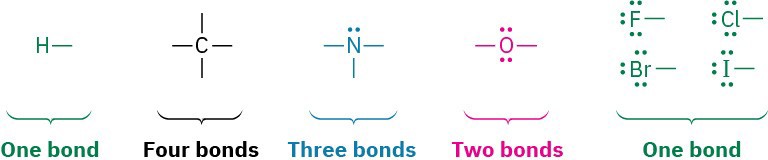
Valence electrons that are not used for bonding remain as dots in structures and are called nonbonding electrons, or more commonly, a lone pair. The nitrogen atom in ammonia, NH3, for instance, shares six valence electrons in three covalent bonds and has its remaining two valence electrons as two dots in a nonbonding lone pair.
Worked Example 1.1 Predicting the Number of Bonds Formed by Atoms in Molecules
How many hydrogen atoms does phosphorus bond to in forming phosphine, PH??
Strategy. Identify the periodic group of phosphorus, and find from that how many electrons (bonds) are needed to make an octet.
Solution. Phosphorus is in group 5A of the periodic table and has five valence electrons. It thus needs to share three more electrons to make an octet and therefore bonds to three hydrogen atoms, giving PH3.
Worked Example 1.2 Drawing Electron-Dot and Line-Bond Structures
Draw both electron-dot and line-bond structures for chloromethane, CH3Cl.
Strategy. Remember that a covalent bond—that is, a pair of shared electrons—is represented as a line between atoms.
Solution. Hydrogen has one valence electron, carbon has four valence electrons, and chlorine has seven valence electrons. Thus, chloromethane is represented as

Problem 1-3
Draw a molecule of chloroform, CHCl3, using solid, wedged, and dashed lines to show its tetrahedral geometry.
Problem 1-4
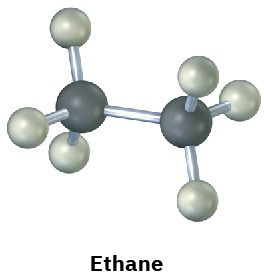
Convert the following representation of ethane, C2H6, into a conventional drawing that uses solid, wedged, and dashed lines to indicate tetrahedral geometry around each carbon (black = C, gray = H).
Problem 1-5
What are likely formulas for the following substances?
(a) CCl?
(b) AlH?
(c) CH?Cl2
(d) SiF?
(e) CH3NH?
Problem 1-6
Write line-bond structures for the following substances, showing all nonbonding electrons:
(a) CHCl3, chloroform
(b) H2S, hydrogen sulfide
(c) CH3NH2, methylamine
(d) CH3Li, methyllithium
Problem 1-7
Why can’t an organic molecule have the formula C2H7?

