In acidic solution, at low pH, a carboxylic acid is completely undissociated and exists entirely as RCOH. In basic solution, at high pH, a carboxylic acid is completely dissociated and exists entirely as RCO2–. Inside living cells, however, the pH is neither acidic nor basic but is instead buffered to a nearly neutral pH of 3.5–4.5 in humans, a value often referred to as physiological pH. In what form, then, do carboxylic acids exist inside cells? The question is an important one for understanding the acid catalysts so often found in biological reactions.
If the pKa value of a given acid and the pH of the medium are known, the percentages of dissociated and undissociated forms can be calculated using the Henderson–Hasselbalch equation.
For any acid HA, we have
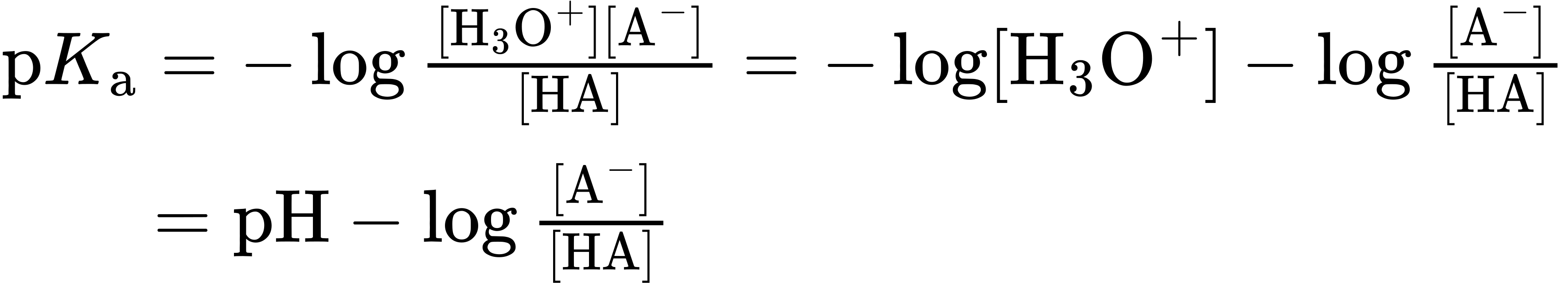
which can be rearranged to give
[A!]
pH = p𝐾a + log [HA]
Henderson-Hasselbach equation
so
[A!]
log [HA] = pH − p𝐾a
This equation says that the logarithm of the concentration of dissociated acid [A–] divided by the concentration of undissociated acid [HA] is equal to the pH of the solution minus the pKa of the acid. Thus, if we know both the pH of the solution and the pKa of the acid, we can calculate the ratio of [A–] to [HA]. Furthermore, when pH = pKa, then HA and A– are present in equal amounts because log 1 = 0.
As an example of how to use the Henderson–Hasselbalch equation, let’s find out what species are present in a 0.0010 M solution of acetic acid at pH = 7.3. According to Table 20.3, the pKa of acetic acid is 4.76. From the Henderson–Hasselbalch equation, we have
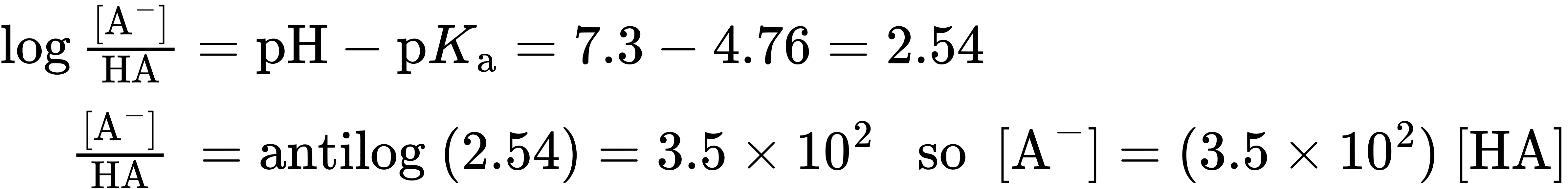
In addition, we know that
[A–] + [HA] = 0.0010 M
Solving the two simultaneous equations gives [A–] = 0.0010 M and [HA] = 3 × 10–6 M. In other words, at a physiological pH of 7.3, essentially 100% of acetic acid molecules in a 0.0010 M solution are dissociated to the acetate ion.
What is true for acetic acid is also true for other carboxylic acids: At the physiological pH that occurs inside cells, carboxylic acids are almost entirely dissociated. To reflect this fact, we always refer to cellular carboxylic acids by the name of their anion—acetate, lactate, citrate, and so forth, rather than acetic acid, lactic acid, and citric acid.
Problem 20-5
Calculate the percentages of dissociated and undissociated forms present in the following solutions:
(a) 0.0010 M glycolic acid (HOCH2CO2H; pKa = 3.83) at pH = 4.50
(b) 0.0020 M propanoic acid (pKa = 4.87) at pH = 5.30

