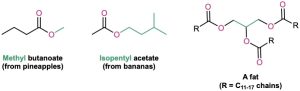
Esters are among the most widespread of all naturally occurring compounds. Many simple esters are pleasant-smelling liquids that are responsible for the fragrant odors of fruits and flowers. For example, methyl butanoate is found in pineapple oil, and isopentyl acetate is a constituent of banana oil. The ester linkage is also present in animal fats and in many biologically important molecules.
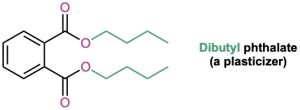
The chemical industry uses esters for a variety of purposes. Ethyl acetate, for instance, is a commonly used solvent, and dialkyl phthalates are used as plasticizers to keep polymers from becoming brittle. You may be aware that there is current concern about the possible toxicity of phthalates at high concentrations, although a recent assessment by the U.S. Food and Drug Administration found the risk to be minimal for most people, with the possible exception of male infants.
Preparation of Esters
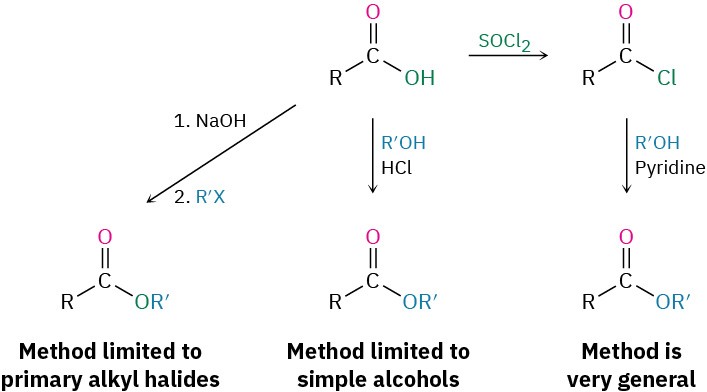
Esters are usually prepared from carboxylic acids by the methods already discussed. Thus, carboxylic acids are converted directly into esters by SN2 reaction of a carboxylate ion with a primary alkyl halide or by Fischer esterification of a carboxylic acid with an alcohol in the presence of a mineral acid catalyst. In addition, acid chlorides are converted into esters by treatment with an alcohol in the presence of base (Section 21.4).
Reactions of Esters
Esters undergo the same kinds of reactions that we’ve seen for other carboxylic acid derivatives, but they are less reactive toward nucleophiles than either acid chlorides or anhydrides. All their reactions are applicable to both acyclic and cyclic esters, called lactones.
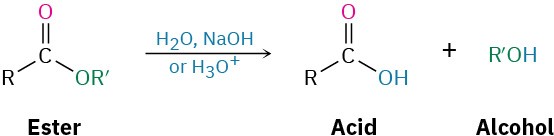
Conversion of Esters into Carboxylic Acids: Hydrolysis
An ester is hydrolyzed, either by aqueous base or aqueous acid, to yield a carboxylic acid plus an alcohol.
Ester hydrolysis in basic solution is called saponification, after the Latin word sapo, meaning “soap.” We’ll see in Section 27.2 that soap is in fact made by boiling animal fat with aqueous base to hydrolyze the ester linkages.
As shown in Figure 21.8, ester hydrolysis occurs through a typical nucleophilic acyl substitution pathway in which hydroxide ion is the nucleophile that adds to the ester carbonyl group to give a tetrahedral intermediate. Loss of alkoxide ion then gives a carboxylic acid, which is deprotonated to give the carboxylate ion. Addition of aqueous HCl, in a separate step after the saponification is complete, protonates the carboxylate ion and gives the carboxylic acid.
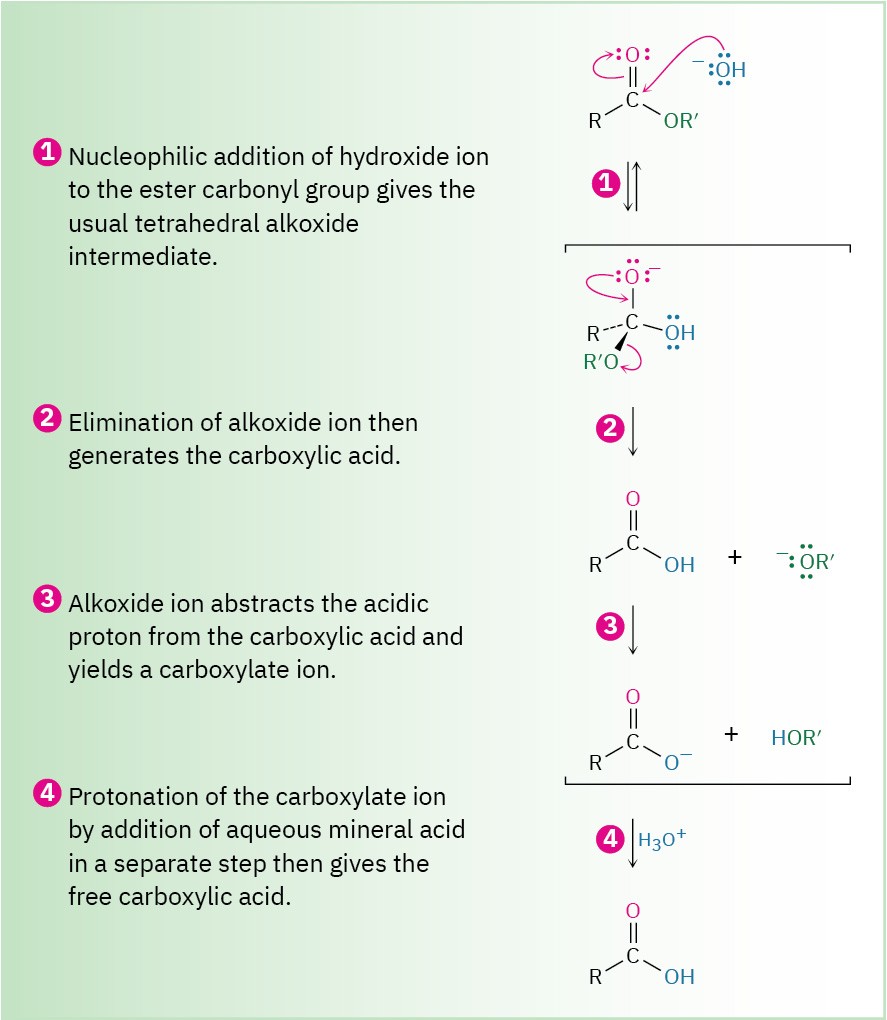
Figure 21.8 Mechanism of base-induced ester hydrolysis (saponification).
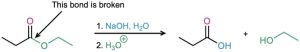
The mechanism shown in Figure 21.8 is supported by isotope-labeling studies. When ethyl propanoate labeled with 18O in the ether-like oxygen is hydrolyzed in aqueous NaOH, the 18O label shows up exclusively in the ethanol product. None of the label remains with the propanoic acid, indicating that saponification occurs by cleavage of the C–OR′ bond rather than the CO–R′ bond.
Acid-catalyzed ester hydrolysis can occur by more than one mechanism, depending on the structure of the ester. The usual pathway, however, is just the reverse of a Fischer esterification reaction (Section 21.3). As shown in Figure 21.9, the ester is first activated toward nucleophilic attack by protonation of the carboxyl oxygen atom, and nucleophilic addition of water then occurs. Transfer of a proton and elimination of alcohol yields the carboxylic acid. Because this hydrolysis reaction is the reverse of a Fischer esterification reaction, Figure 21.9 is the reverse of Figure 21.5.
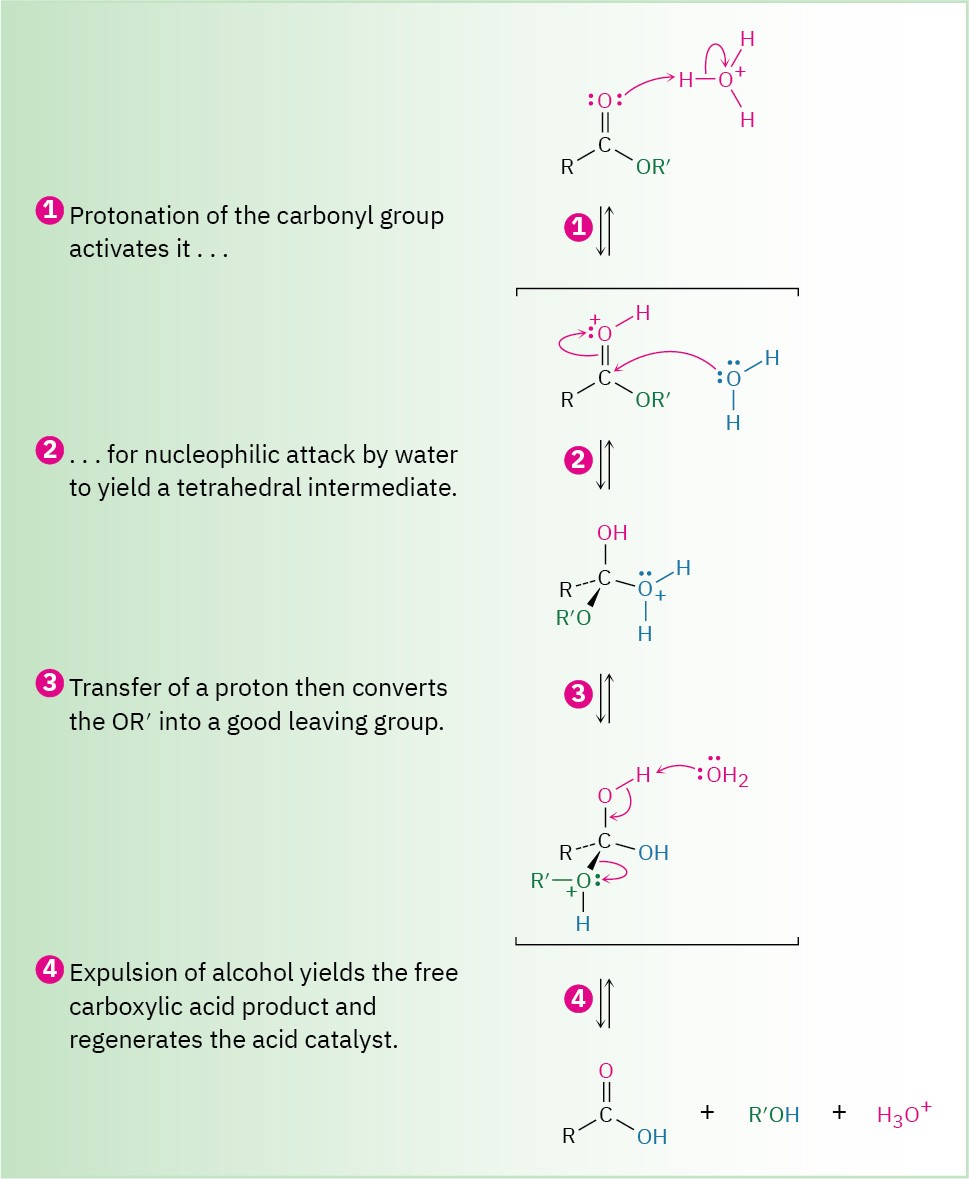
Figure 21.9 Mechanism of acid-catalyzed ester hydrolysis. The forward reaction is a hydrolysis; the back-reaction is a Fischer esterification and is thus the reverse of Figure 21.5.
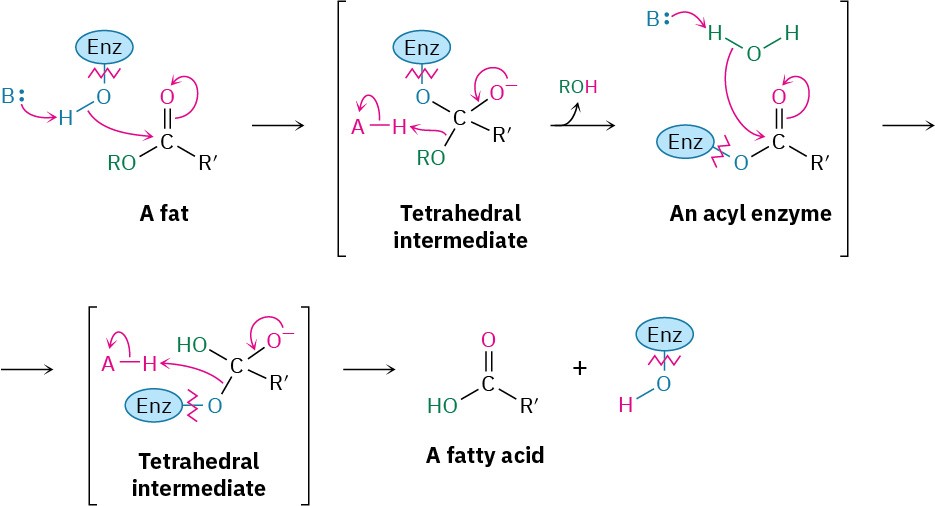
Ester hydrolysis is common in biological chemistry, particularly in the digestion of dietary fats and oils. We’ll save a complete discussion of the mechanistic details of fat hydrolysis until Section 29.2 but will note for now that the reaction is catalyzed by various lipase enzymes and involves two sequential nucleophilic acyl substitution reactions. The first is a transesterification reaction in which an alcohol group on the lipase adds to an ester linkage in the fat molecule to give a tetrahedral intermediate that expels alcohol and forms an acyl enzyme intermediate. The second is an addition of water to the acyl enzyme, followed by expulsion of the enzyme to give a hydrolyzed acid and a regenerated enzyme.
Problem 21-16
Why is the saponification of an ester irreversible? In other words, why doesn’t treatment of a carboxylic acid with an alkoxide ion yield an ester?
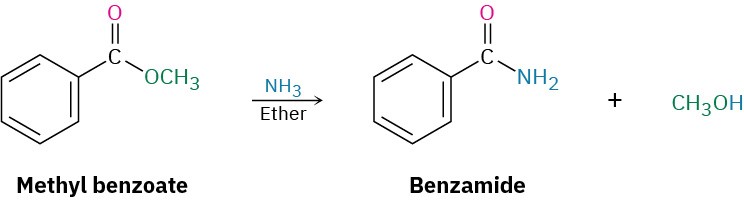
Conversion of Esters into Amides: Aminolysis
Esters react with ammonia and amines to yield amides. The reaction is not often used, however, because it’s usually easier to prepare an amide by starting with an acid chloride (Section 21.4).
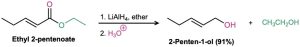
Conversion of Esters into Alcohols: Reduction
Esters are easily reduced by treatment with LiAlH4 to yield primary alcohols (Section 17.4).
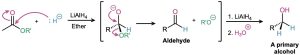
The mechanism of ester reduction is similar to that of acid chloride reduction in that a hydride ion first adds to the carbonyl group, followed by elimination of alkoxide ion to yield an aldehyde. Further reduction of the aldehyde gives the primary alcohol.
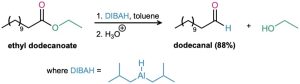
The aldehyde intermediate can be isolated if 1 equivalent of diisobutylaluminum hydride (DIBAH, or DIBAL-H) is used as the reducing agent instead of LiAlH4. The reaction has to be carried out at –78 °C to avoid further reduction to the alcohol. Such partial reductions of carboxylic acid derivatives to aldehydes also occur in numerous biological pathways, although the substrate is either a thioester or acyl phosphate rather than an ester.
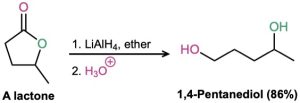
Problem 21-17
What product would you expect from the reaction of butyrolactone with LiAlH4? With DIBAH?

Problem 21-18
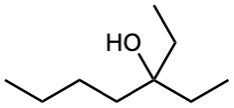
Show the products you would obtain by reduction of the following esters with LiAlH4:
(a)
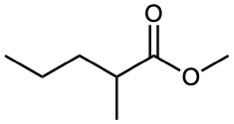
(b)
Conversion of Esters into Alcohols: Grignard Reaction
Esters react with 2 equivalents of a Grignard reagent to yield a tertiary alcohol in which two of the substituents are identical (Section 17.5). The reaction occurs by the usual nucleophilic substitution mechanism to give an intermediate ketone, which reacts further with the Grignard reagent to yield a tertiary alcohol.
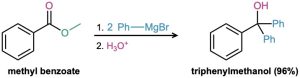
Problem 21-19
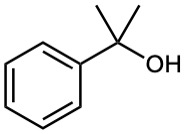
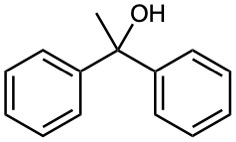
What ester and what Grignard reagent might you start with to prepare the following alcohols?
(a)
(b)
(c)