Infrared Spectroscopy
All carbonyl-containing compounds have intense IR absorptions in the range 1650 to 1850 cm–1. As shown in Table 21.3 the exact position of the absorption provides information about the specific kind of carbonyl group. For comparison, the IR absorptions of aldehydes, ketones, and carboxylic acids are included in the table, along with values for carboxylic acid derivatives.
Table 21.3 Infrared Absorptions of Some Carbonyl Compounds
|
Carbonyl type |
Example |
Absorption (cm–1) |
|
Saturated acid chloride |
Acetyl chloride |
1810 |
|
Aromatic acid chloride |
Benzoyl chloride |
1770 |
|
Saturated acid anhydride |
Acetic anhydride |
1820, 1760 |
|
Saturated ester |
Ethyl acetate |
1735 |
|
Aromatic ester |
Ethyl benzoate |
1720 |
|
Saturated amide |
Acetamide |
1690 |
|
Aromatic amide |
Benzamide |
1675 |
|
N-Substituted amide |
N-Methylacetamide |
1680 |
|
N,N-Disubstituted amide |
N,N-Dimethylacetamide |
1650 |
|
Saturated aldehyde |
Acetaldehyde |
1730 |
|
Saturated ketone |
Acetone |
1715 |
|
Saturated carboxylic acid |
Acetic acid |
1710 |
Acid chlorides are easily detected by their characteristic absorption near 1810 cm–1. Acid anhydrides can be identified by a pair of absorptions in the carbonyl region, one at 1820 cm–1 and another at 1760 cm–1. Note that each of these functional groups has a strong electron-withdrawing group attached to the carbonyl. The inductive withdrawal of electron density shortens the C=O bond, thereby raising its stretching frequency. Esters are detected by their absorption at 1735 cm–1, a position somewhat higher than that for either aldehydes or ketones. Amides, by contrast, absorb near the low-wavenumber end of the carbonyl region, with the degree of substitution on nitrogen affecting the exact position of the IR band. That is, for amides, the delocalization of electron density (resonance) from nitrogen into the carbonyl lengthens the C=O bond and lowers its stretching frequency.
Problem 21-25
What kinds of functional groups might compounds have if they show the following IR absorptions?
(a) Absorption at 1735 cm–1
(b) Absorption at 1810 cm–1
(c) Absorptions at 2500 to 3300 cm–1 and 1710 cm–1
(d) Absorption at 1715 cm–1
Problem 21-26
Propose structures for compounds that have the following formulas and IR absorptions:
(a) C6H12O2, 1735 cm–1
(b) C4H9NO, 1650 cm–1
(c) C4H5ClO, 1780 cm–1
Nuclear Magnetic Resonance Spectroscopy
Hydrogens on the carbon next to a carbonyl group are slightly deshielded and absorb near 2 δ in the 1H NMR spectrum, although the exact identity of the carbonyl group can’t be determined. Figure 21.11 shows the 1H NMR spectrum of ethyl acetate.
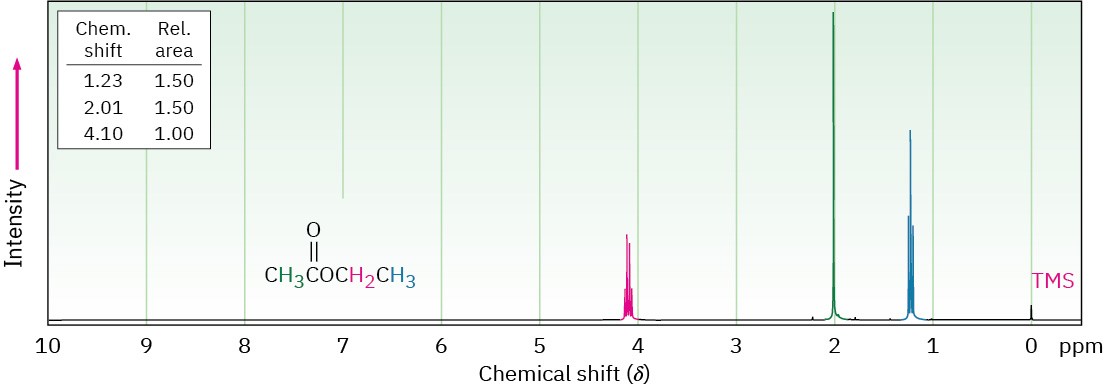
Figure 21.11 1H NMR spectrum of ethyl acetate.
Although 13C NMR is useful for determining the presence or absence of a carbonyl group in a molecule, the identity of the carbonyl group is difficult to determine. Aldehydes and ketones absorb near 200 δ, while the carbonyl carbon atoms of various acid derivatives absorb in the range 160 to 180 δ (Table 21.4).
Table 21.4 13C NMR Absorptions of Some Carbonyl Compounds
|
Compound |
Absorption (δ) |
|
Acetic acid |
177.3 |
|
Ethyl acetate |
170.7 |
|
Acetyl chloride |
170.3 |
|
Acetamide |
172.6 |
|
Acetic anhydride |
166.9 |
|
Acetone |
205.6 |
|
Acetaldehyde |
201.0 |

