Just as waxes, fats, and oils are esters of carboxylic acids, phospholipids are esters of phosphoric acid, H3PO4.
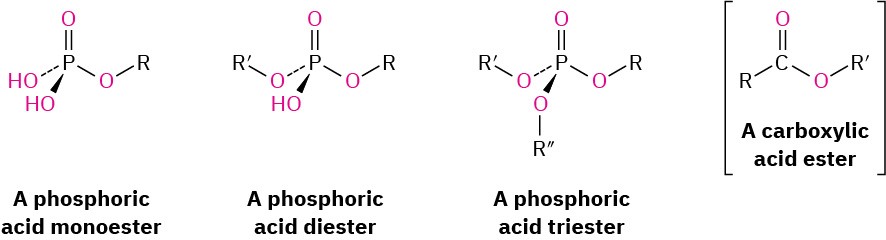
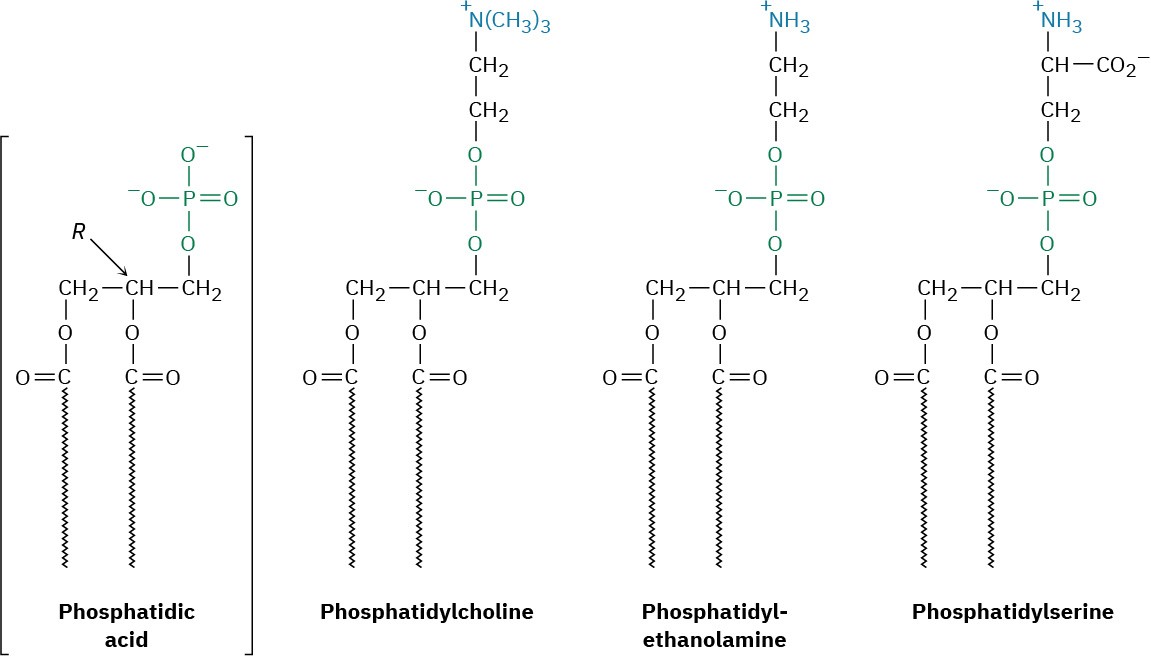
Phospholipids are of two general types: glycerophospholipids and sphingomyelins. Glycerophospholipids are based on phosphatidic acid, which contains a glycerol backbone linked by ester bonds to two fatty acids and one phosphoric acid. Although the fatty-acid residues can be any of the C12–C20 units typically present in fats, the acyl group at C1 is usually saturated and the one at C2 is usually unsaturated. The phosphate group at C3 is also bonded to an amino alcohol such as choline [HOCH2CH2N(CH3)3]+, ethanolamine (HOCH2CH2NH2), or serine [HOCH2CH(NH2)CO2H]. The compounds are chiral and have an L, or R, configuration at C2.
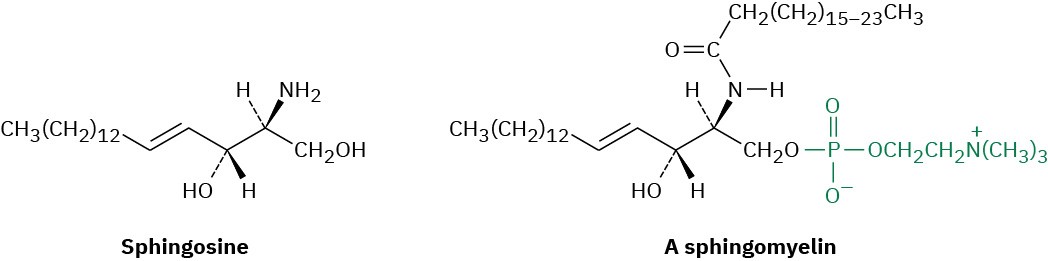
Sphingomyelins are the second major group of phospholipids. These compounds have sphingosine or a related dihydroxyamine as their backbone and are particularly abundant in brain and nerve tissue, where they are a major constituent of the coating around nerve fibers.
Phospholipids are found widely in both plant and animal tissues and make up approximately 50% to 60% of cell membranes. Because they are like soaps in having a long, nonpolar hydrocarbon tail bound to a polar ionic head, phospholipids in the cell membrane organize into a lipid bilayer about 5.0 nm (50 Å) thick. As shown in Figure 27.3, the nonpolar tails aggregate in the center of the bilayer in much the same way that soap tails aggregate in the center of a micelle. This bilayer serves as an effective barrier to the passage of water, ions, and other components into and out of cells.
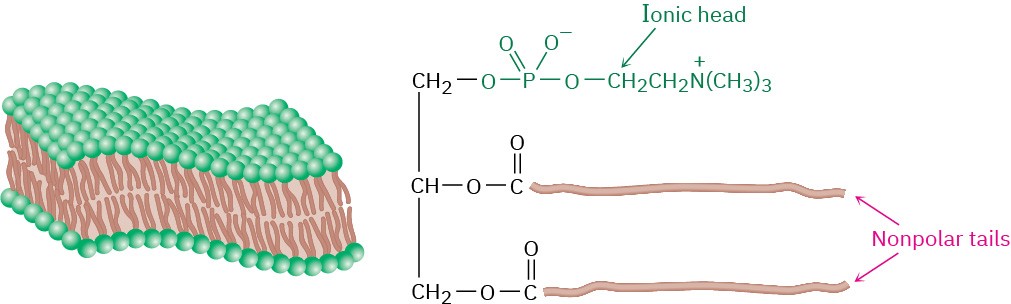
Figure 27.3Aggregation of glycerophospholipids into the lipid bilayer that composes cell membranes.

