Glucose is the body’s primary short-term energy source. Its catabolism begins with glycolysis, a series of ten enzyme-catalyzed reactions that break down glucose into 2 equivalents of pyruvate, CH3COCO2–. The steps of glycolysis, also called the Embden– Meyerhoff pathway after its discoverers, are summarized in Figure 29.8.
Figure 29.8 MECHANISM
The ten-step glycolysis pathway for catabolizing glucose to two molecules of pyruvate. Individual steps are described in the text.
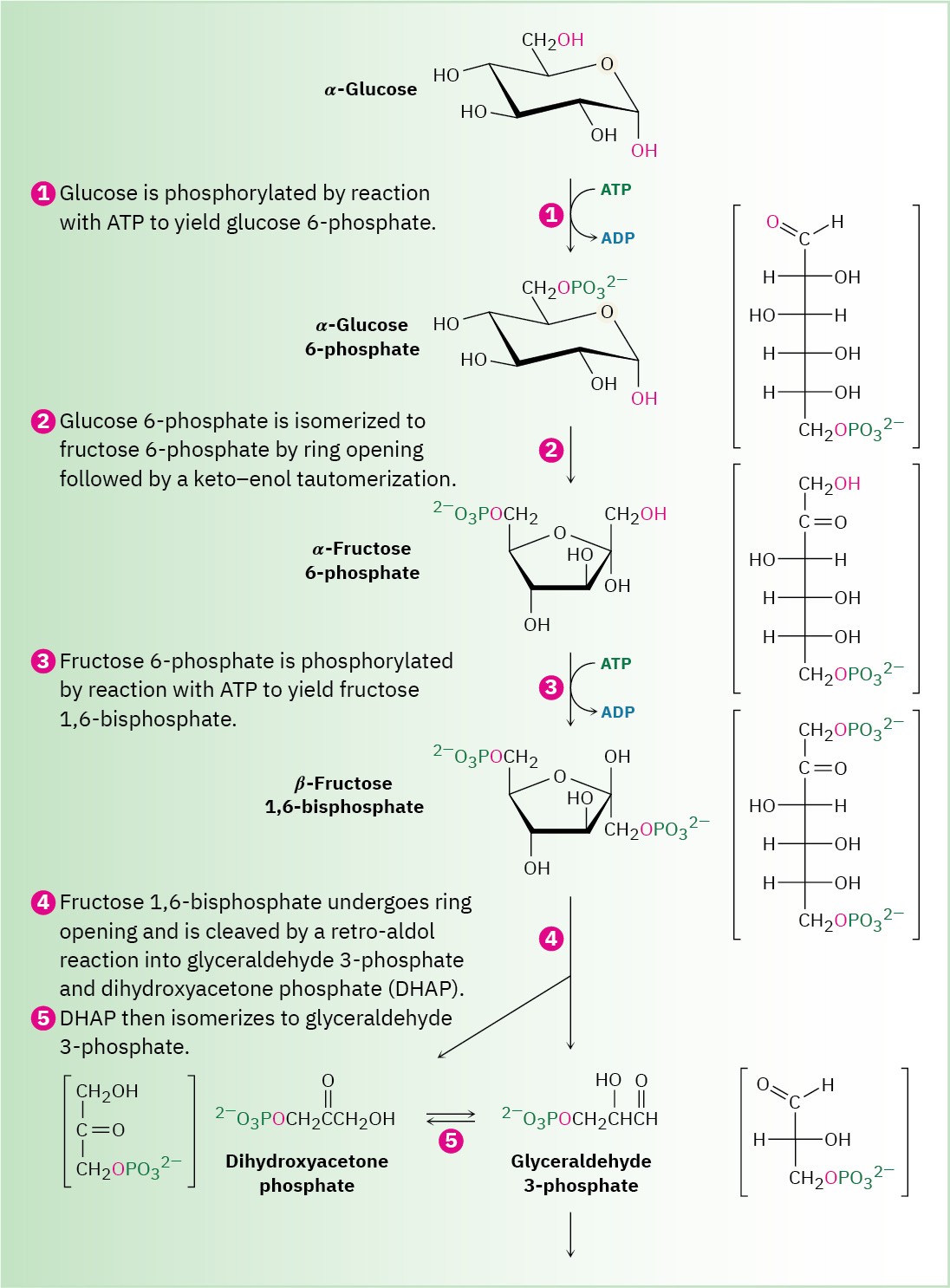
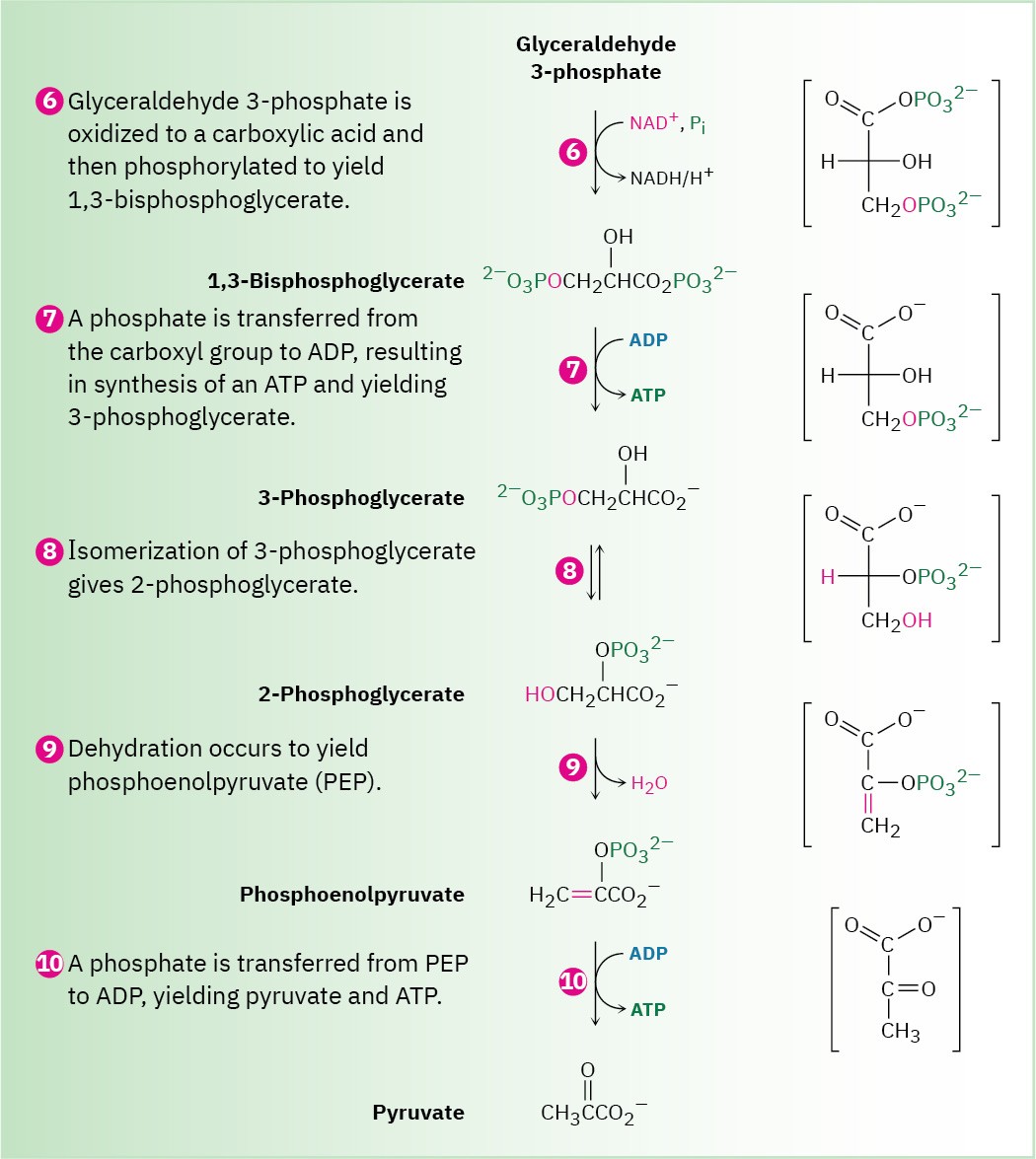
Figure 29.9 MECHANISM
(Continued)
Steps 1–2 of Figure 29.8: Phosphorylation and Isomerization
Glucose, produced by the digestion of dietary carbohydrates, is phosphorylated at the C6 hydroxyl group by reaction with ATP in a process catalyzed by hexokinase. As noted in Section 29.1, the reaction requires Mg2+ as a cofactor to complex with the negatively charged phosphate oxygens. The glucose 6-phosphate that results is then isomerized by glucose 6-phosphate isomerase to give fructose 6-phosphate. This isomerization takes place by initial opening of the glucose hemiacetal ring to its open-chain form, followed by keto–enol tautomerization to a cis enediol, HO─C═C─OH. But because glucose and fructose share a common enediol, further tautomerization to a different keto form produces open- chain fructose, and cyclization completes the process (Figure 29.10).
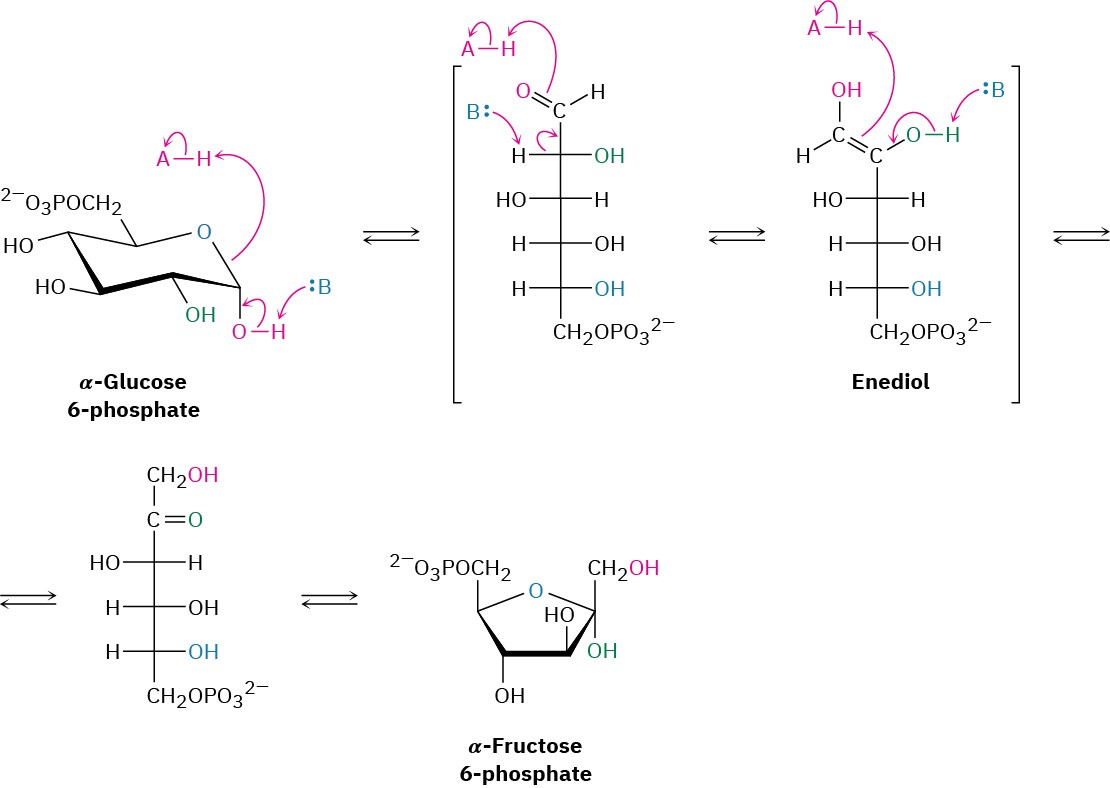
Figure 29.10Mechanism of step 2 in glycolysis, the isomerization of glucose 6- phosphate to fructose 6-phosphate.
Step 3 of Figure 29.8: Phosphorylation
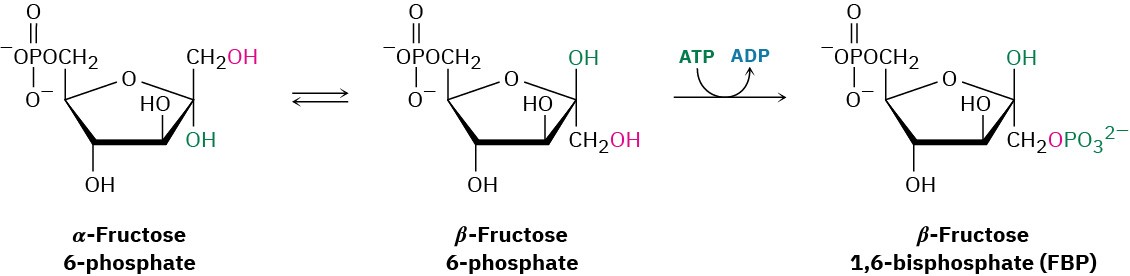
Fructose 6-phosphate is converted in step 3 to fructose 1,6-bisphosphate (FBP) by a phosphofructokinase-catalyzed reaction with ATP (recall that the prefix bis– means two). The mechanism is similar to that in step 1, with Mg2+ ion again required as cofactor.
Interestingly, the product of step 2 is the α anomer of fructose 6-phosphate, but it is the β anomer that is phosphorylated in step 3, implying that the two anomers equilibrate rapidly through the open-chain form. The result is a molecule ready to be split into the two three- carbon intermediates that will ultimately become two molecules of pyruvate.
Step 4 of Figure 29.8: Cleavage
Fructose 1,6-bisphosphate is cleaved in step 4 into two 3-carbon pieces, dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (GAP). The bond between C3 and C4 of fructose 1,6-bisphosphate breaks, and a C═O group is formed at C4. Mechanistically, the
cleavage is the reverse of an aldol reaction (Section 23.1) and is catalyzed by an aldolase. A forward aldol reaction joins two aldehydes or ketones to give a β-hydroxy carbonyl compound, while a retro-aldol reaction, as in this case, cleaves a β-hydroxy carbonyl compound into two aldehydes or ketones.
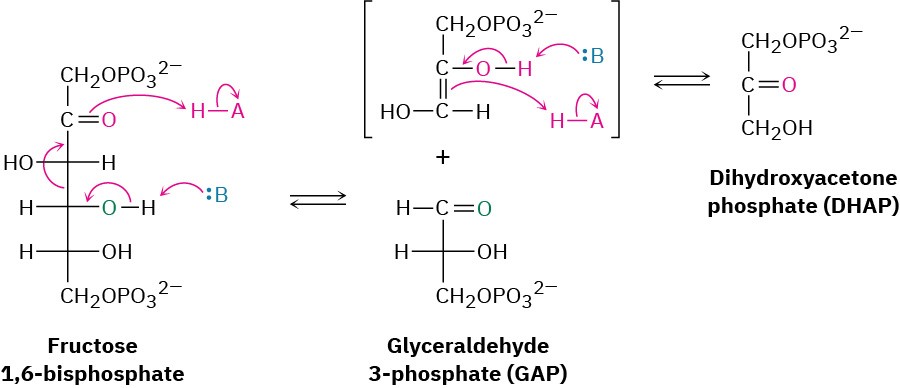
Two classes of aldolases are used by organisms for catalysis of the retro-aldol reaction. In fungi, algae, and some bacteria, the retro-aldol reaction is catalyzed by class II aldolases, which function by coordination of the fructose carbonyl group with Zn2+ as Lewis acid. In plants and animals, the reaction is catalyzed by class I aldolases and does not take place on the free ketone. Instead, fructose 1,6-bisphosphate undergoes reaction with the side-chain
–NH2 group of a lysine residue on the aldolase to yield a protonated enzyme-bound imine (Section 19.8), which is often called a Schiff base in biochemistry.
Because of its positive charge, the iminium ion is a better electron acceptor than a ketone carbonyl group. Retro-aldol reaction ensues, giving glyceraldehyde 3-phosphate and an enamine, which is protonated to give another iminium ion that is hydrolyzed to yield dihydroxyacetone phosphate (Figure 29.11).
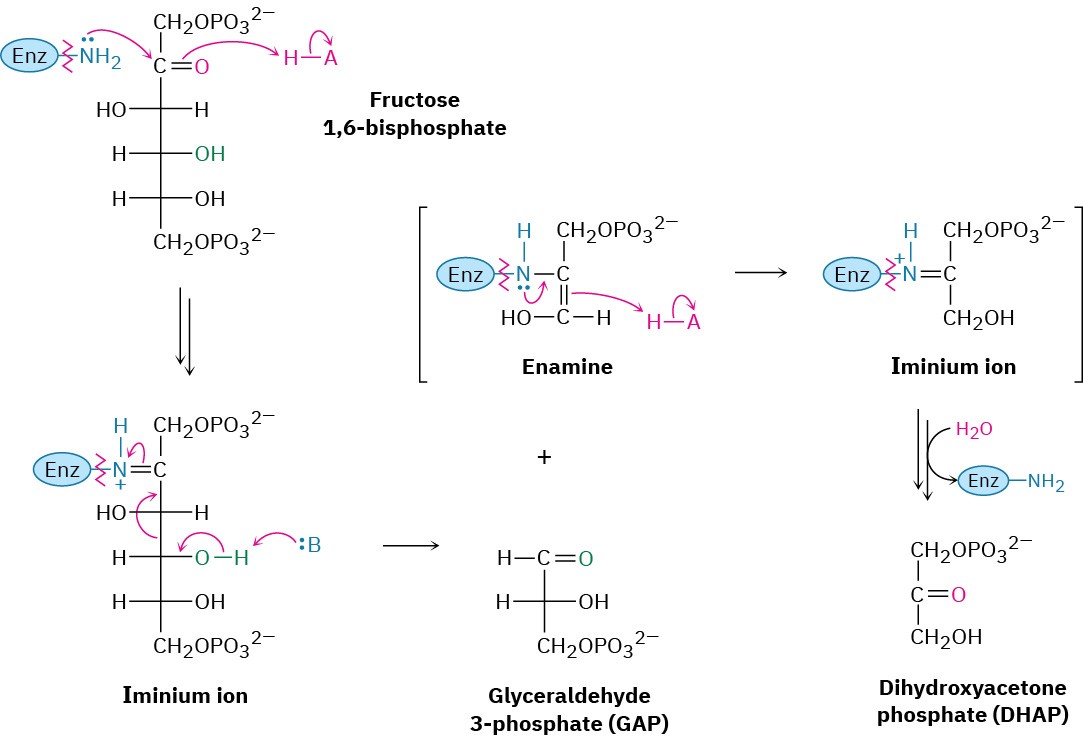
Figure 29.11 Mechanism of step 4 in Figure 29.8, the cleavage of fructose 1,6- bisphosphate to yield glyceraldehyde 3-phosphate and dihydroxyacetone phosphate. The reaction occurs through an iminium ion formed by reaction with a lysine residue in the enzyme.
Step 5 of Figure 29.8: Isomerization
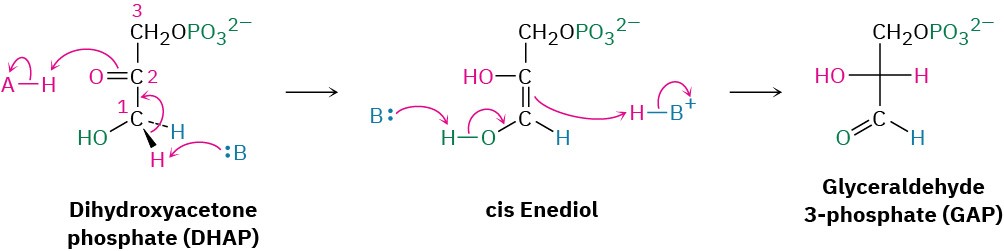
Dihydroxyacetone phosphate is isomerized in step 5 by triose phosphate isomerase to form a second equivalent of glyceraldehyde 3-phosphate. As in the conversion of glucose 6- phosphate to fructose 6-phosphate in step 2, the isomerization takes place by keto–enol tautomerization through a common enediol intermediate. A base deprotonates C1 and then reprotonates C2 using the same hydrogen. The net result of steps 4 and 5 together is the production of two glyceraldehyde 3-phosphate molecules, both of which pass down the rest of the pathway. Thus, each of the remaining five steps of glycolysis takes place twice for every glucose molecule entering at step 1.
Steps 6–7 of Figure 29.9: Oxidation, Phosphorylation, and Dephosphorylation
Glyceraldehyde 3-phosphate is oxidized and phosphorylated in step 6 to give 1,3- bisphosphoglycerate (Figure 29.12). The reaction is catalyzed by glyceraldehyde 3-
phosphate dehydrogenase and begins by nucleophilic addition of the –SH group of a cysteine residue in the enzyme to the aldehyde carbonyl group to yield a hemithioacetal (Section 19.10), the sulfur analog of a hemiacetal. Oxidation of the hemithioacetal –OH group by NAD+ then yields a thioester, which reacts with phosphate ion in a nucleophilic acyl substitution step to yield 1,3-bisphosphoglycerate, a mixed anhydride derived from a carboxylic acid and a phosphoric acid.
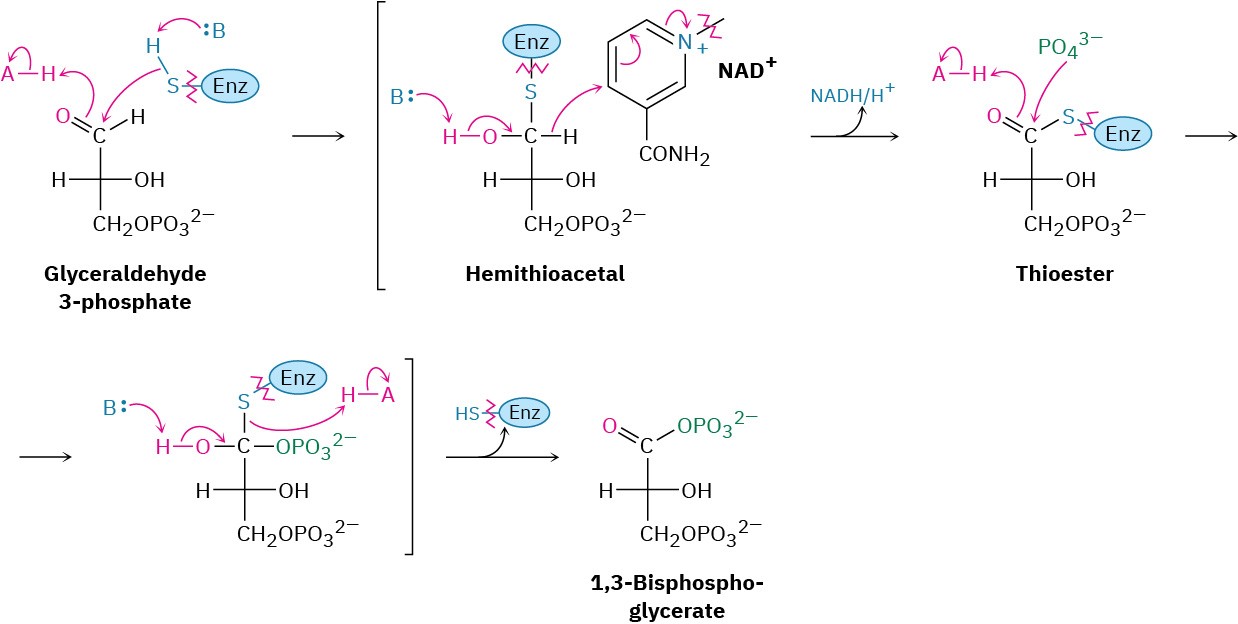
Figure 29.12 Mechanism of step 6 in Figure 29.9, the oxidation and phosphorylation of glyceraldehyde 3-phosphate to give 1,3-bisphosphoglycerate. The process occurs through initial formation of a hemiacetal that is oxidized to a thioester and converted into an acyl phosphate.
Like all anhydrides (Section 21.5), the mixed carboxylic–phosphoric anhydride is a reactive substrate in nucleophilic acyl (or phosphoryl) substitution reactions. Reaction of 1,3- bisphosphoglycerate with ADP occurs in step 7 by substitution on phosphorus, resulting in transfer of a phosphate group to ADP and giving ATP plus 3-phosphoglycerate. The process is catalyzed by phosphoglycerate kinase and requires Mg2+ as cofactor. Together, steps 6 and 7 accomplish the oxidation of an aldehyde to a carboxylic acid.
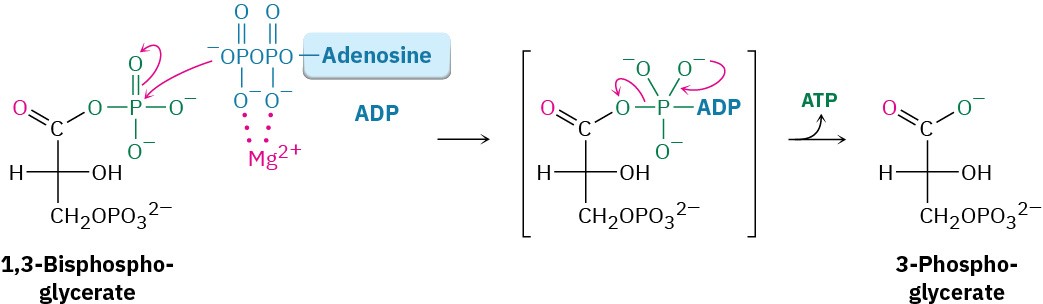
Step 8 of Figure 29.9: Isomerization
3-Phosphoglycerate isomerizes to 2-phosphoglycerate in a step catalyzed by phosphoglycerate mutase. In plants, 3-phosphoglycerate transfers its phosphoryl group from its C3 oxygen to a histidine residue on the enzyme in one step and then accepts the same phosphoryl group back onto the C2 oxygen in a second step. In animals and yeast, however, the enzyme contains a phosphorylated histidine, which transfers its phosphoryl group to the C2 oxygen of 3-phosphoglycerate and forms 2,3-bisphosphoglycerate as intermediate. The same histidine then accepts a phosphoryl group from the C3 oxygen to yield the isomerized product plus the regenerated enzyme. As explained in Section 29.4, we’ll occasionally use an abbreviated mechanism for nucleophilic acyl substitution reactions to save space.
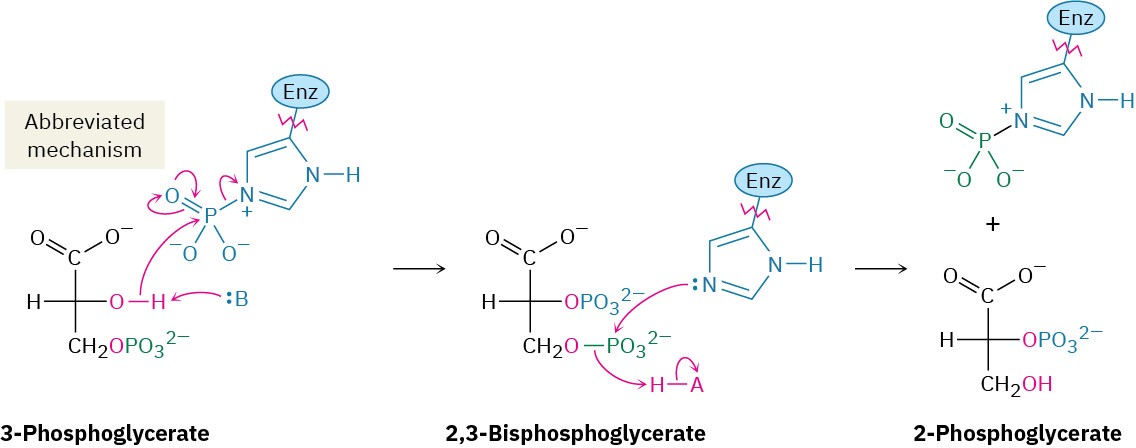
Steps 9–10 of Figure 29.9: Dehydration and Dephosphorylation
Like most β-hydroxy carbonyl compounds, 2-phosphoglycerate undergoes a ready dehydration in step 9 by an E1cB mechanism (Section 23.3). The process is catalyzed by enolase, and the product is phosphoenolpyruvate, abbreviated PEP. Two Mg2+ ions are associated with the 2-phosphoglycerate to neutralize the negative charges.
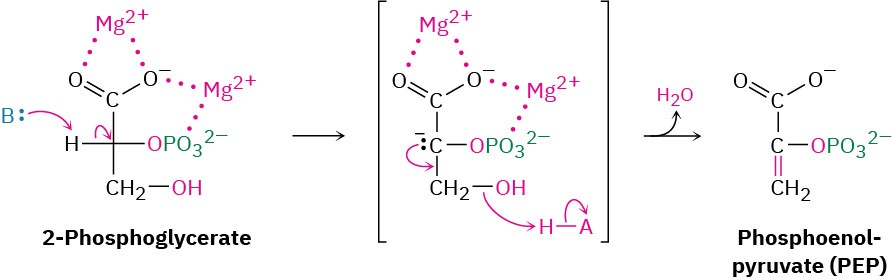
Transfer of the phosphoryl group to ADP in step 10 then generates ATP and gives enolpyruvate, which tautomerizes to pyruvate. The reaction is catalyzed by pyruvate kinase and requires that a molecule of fructose 1,6-bisphosphate also be present, as well as 2 equivalents of Mg2+. One Mg2+ ion coordinates to ADP, and the other increases the acidity of a water molecule necessary for protonation of the enolate ion.
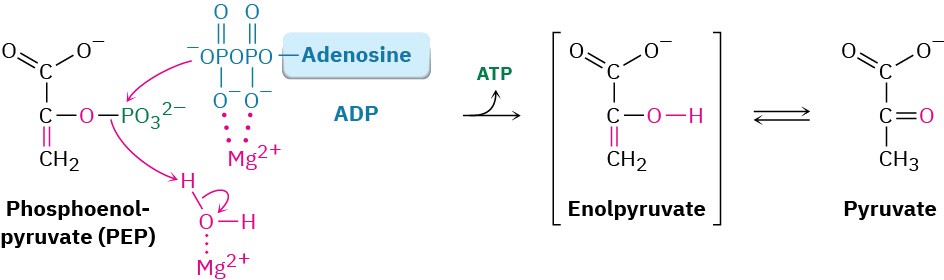
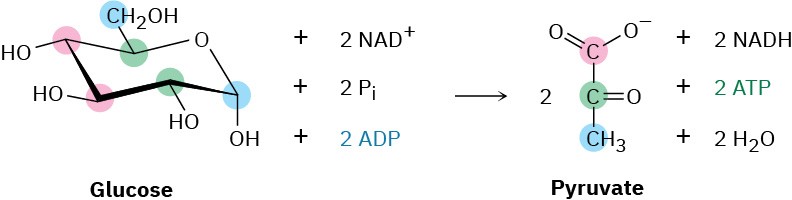
The overall result of glycolysis can be summarized by the following equation:
Problem 29-7
Identify the two steps of glycolysis in which ATP is produced. Problem 29-8
Look at the entire glycolysis pathway, and make a list of the kinds of organic reactions that take place—nucleophilic acyl substitutions, aldol reactions, E1cB reactions, and so forth.

