Visualizing Chemistry
Problem 29-17
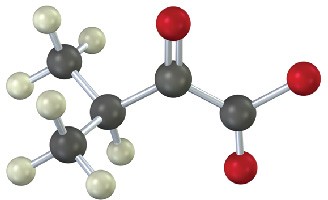
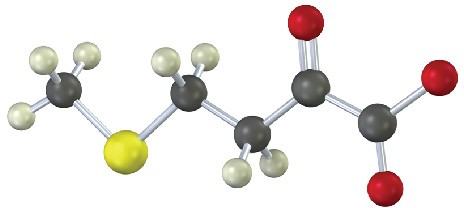
Identify the amino acid that is a catabolic precursor of each of the following α-keto acids:
(a)
(b)
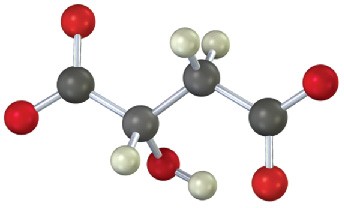
Problem 29-18
Identify the following intermediate in the citric acid cycle, and tell whether it has R or S stereochemistry:
Problem 29-19
The following compound is an intermediate in the biosynthesis of one of the 20 common α– amino acids. Which one is it likely to be, and what kind of chemical change must take place to complete the biosynthesis?
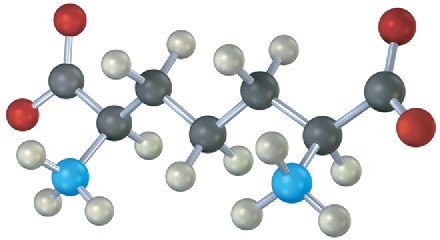
Problem 29-20
The following compound is an intermediate in the pentose phosphate pathway, an alternative route for glucose metabolism. Identify the sugar it is derived from.
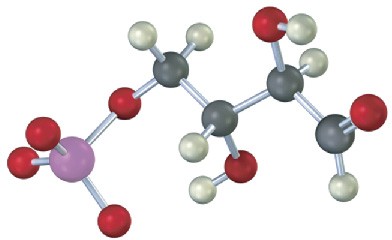
Mechanism Problems
Problem 29-21
In the pentose phosphate pathway for degrading sugars, ribulose 5-phosphate is converted to ribose 5-phosphate. Propose a mechanism for the isomerization.
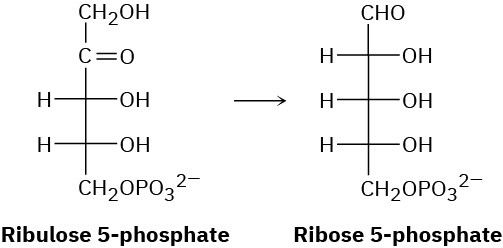
Problem 29-22
Another step in the pentose phosphate pathway for degrading sugars (see Problem 29-21) is the conversion of ribose 5-phosphate to glyceraldehyde 3-phosphate. What kind of organic process is occurring? Propose a mechanism for the conversion.
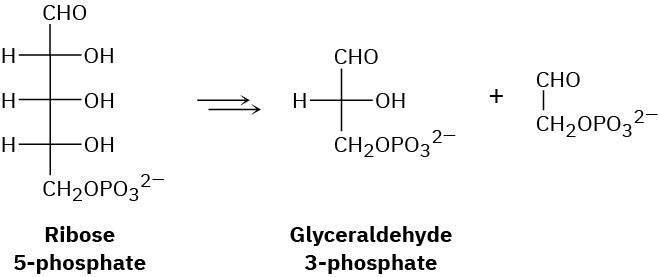
Problem 29-23
One of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of sedoheptulose 7-phosphate with glyceraldehyde 3-phosphate in the presence of a transaldolase to yield erythrose 4-phosphate and fructose 6-phosphate.
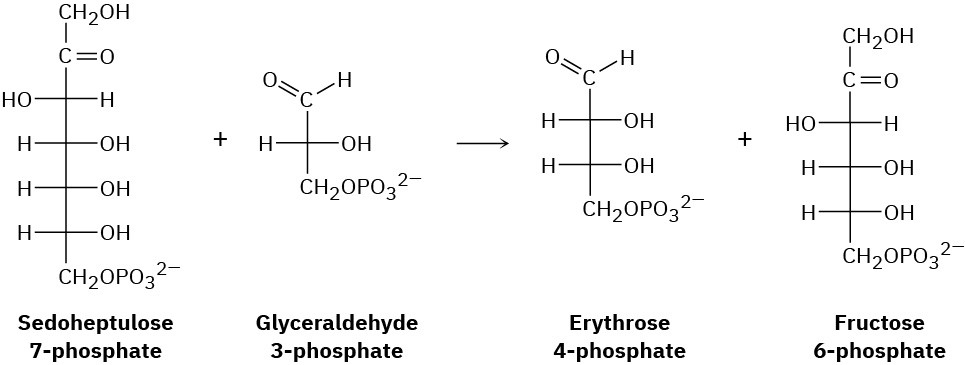
(a) The first part of the reaction is the formation of a protonated Schiff base of sedoheptulose 7-phosphate with a lysine residue in the enzyme followed by a retro-aldol cleavage to give an enamine plus erythrose 4-phosphate. Show the structure of the enamine and the mechanism by which it is formed.
(b) The second part of the reaction is a nucleophilic addition of the enamine to glyceraldehyde 3-phosphate followed by hydrolysis of the Schiff base to give fructose 6-phosphate. Show the mechanism.
Problem 29-24
One of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of xylulose 5-phosphate with ribose 5-phosphate in the presence of a transketolase to give glyceraldehyde 3-phosphate and sedoheptulose 7-phosphate.
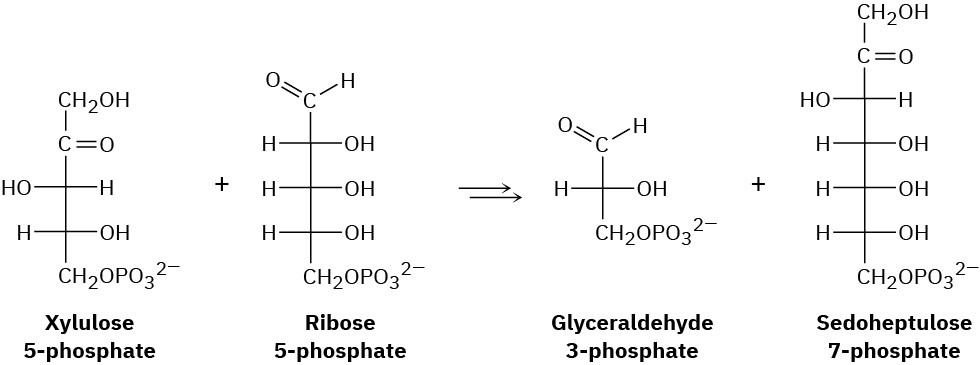
(a) The first part of the reaction is nucleophilic addition of thiamin diphosphate (TPP) ylide to xylulose 5-phosphate, followed by a retro-aldol cleavage to give glyceraldehyde 3-phosphate and a TPP-containing enamine. Show the structure of the enamine and the mechanism by which it is formed.
(b) The second part of the reaction is addition of the enamine to ribose 5-phosphate followed by loss of TPP ylide to give sedoheptulose 7-phosphate. Show the mechanism.
Problem 29-25
The amino acid tyrosine is biologically degraded by a series of steps that include the following transformations:
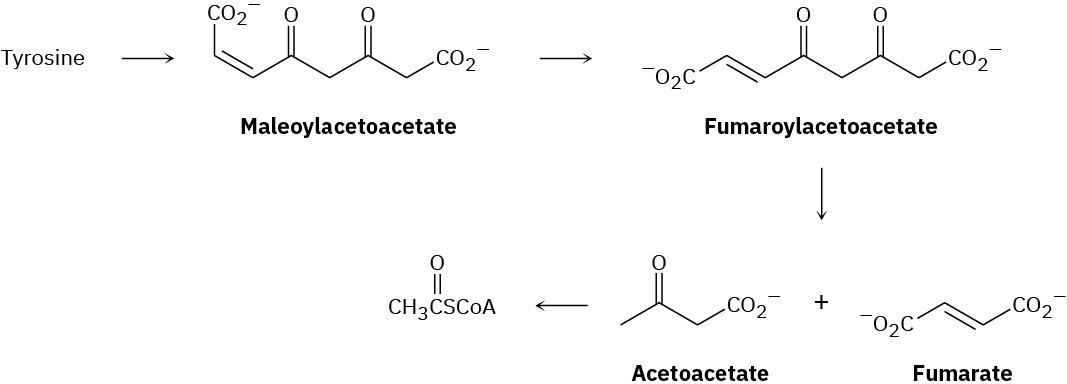
The double-bond isomerization of maleoylacetoacetate to fumaroylacetoacetate is catalyzed by practically any nucleophile, :Nu–. Propose a mechanism.
Problem 29-26
Propose a mechanism for the conversion of fumaroylacetoacetate to fumarate plus acetoacetate (see Problem 29-25).
Problem 29-27
Propose a mechanism for the conversion of acetoacetate to acetyl CoA (see Problem 29-25).
Problem 29-28
Design your own degradative pathway. You know the rules (organic mechanisms), and you’ve seen the kinds of reactions that occur in the biological degradation of fats and carbohydrates into acetyl CoA. If you were Mother Nature, what series of steps would you use to degrade the amino acid serine into acetyl CoA?
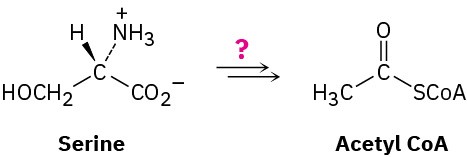
Problem 29-29
The amino acid serine is biosynthesized by a route that involves reaction of 3- phosphohydroxypyruvate with glutamate to give 3-phosphoserine. Propose a mechanism.
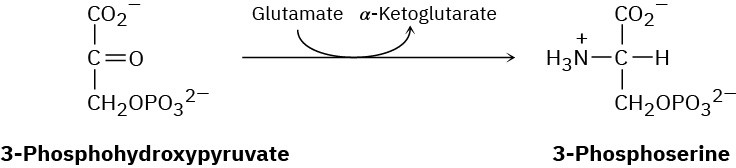
Problem 29-30
The amino acid leucine is biosynthesized from α-ketoisocaproate, which is itself prepared from α-ketoisovalerate by a multistep route that involves (1) reaction with acetyl CoA, (2) hydrolysis, (3) dehydration, (4) hydration, (5) oxidation, and (6) decarboxylation. Show the steps in the transformation, and propose a mechanism for each.
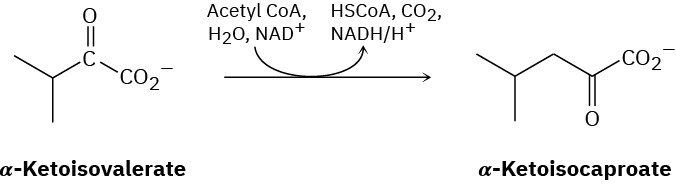
Problem 29-31
The amino acid cysteine, C3H7NO2S, is biosynthesized from a substance called cystathionine by a multistep pathway.
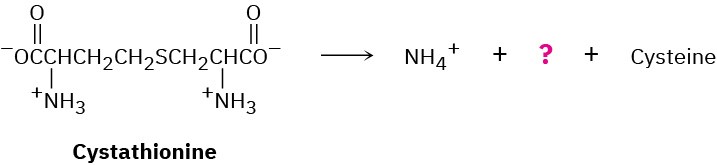
(a) The first step is a transamination. What is the product?
(b) The second step is an E1cB reaction. Show the products and the mechanism of the reaction.
(c) The final step is a double-bond reduction. What organic cofactor is required for this reaction, and what is the product represented by the question mark in the equation?
Enzymes and Coenzymes
Problem 29-32
What chemical events occur during the digestion of food?
Problem 29-33
What is the difference between digestion and metabolism?
Problem 29-34
What is the difference between anabolism and catabolism?
Problem 29-35
Draw the structure of adenosine 5′-monophosphate (AMP), an intermediate in some biochemical pathways.
Problem 29-36
Cyclic adenosine monophosphate (cyclic AMP), a modulator of hormone action, is related to AMP (Problem 29-35) but has its phosphate group linked to two hydroxyl groups at C3′ and C5′ of the sugar. Draw the structure of cyclic AMP.
Problem 29-37
What general kind of reaction does ATP carry out?
Problem 29-38
What general kind of reaction does NAD+ carry out?
Problem 29-39
What general kind of reaction does FAD carry out?
Problem 29-40
What enzyme cofactor is associated with each of the following kinds of reactions?
(a) Transamination
(b) Carboxylation of a ketone
(c) Decarboxylation of an α-keto acid
Problem 29-41
Lactate, a product of glucose catabolism in oxygen-starved muscles, can be converted into pyruvate by oxidation. What coenzyme do you think is needed? Write the equation in the normal biochemical format using a curved arrow.
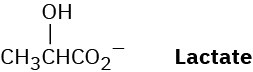
Metabolism
Problem 29-42
Write the equation for the final step in the β-oxidation pathway of any fatty acid with an even number of carbon atoms.
Problem 29-43
Show the products of each of the following reactions:
(a)
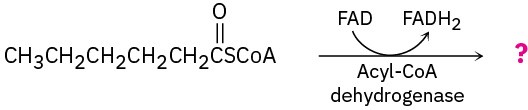
(b)
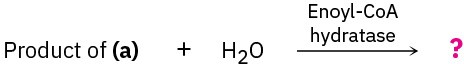
(c)
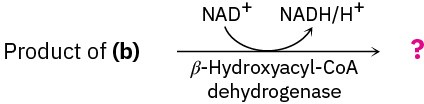
Problem 29-44
Why aren’t the glycolysis and gluconeogenesis pathways the exact reverse of each other?
Problem 29-45
How many moles of acetyl CoA are produced by catabolism of the following substances?
(a) 1.0 mol of glucose
(b) 1.0 mol of palmitic acid
(c) 1.0 mol of maltose
Problem 29-46
How many grams of acetyl CoA (MW = 809.6 amu) are produced by catabolism of the following substances? Which substance is the most efficient precursor of acetyl CoA on a weight basis?
(a) 100.0 g of glucose
(b) 100.0 g of palmitic acid
(c) 100.0 g of maltose
Problem 29-47
What is the structure of the α-keto acid formed by transamination of each of the following amino acids?
(a) Threonine
(b) Phenylalanine
(c) Asparagine
Problem 29-48
The glycolysis pathway shown in Figure 29.8 has a number of intermediates that contain phosphate groups. Why can 3-phosphoglyceryl phosphate and phosphoenolpyruvate transfer a phosphate group to ADP while glucose 6-phosphate cannot?
Problem 29-49
Write a mechanism for the conversion of α-ketoglutarate to succinyl CoA in step 4 of the citric acid cycle (Figure 29.14).
Problem 29-50
In step 2 of the citric acid cycle (Figure 29.14), cis-aconitate reacts with water to give (2R,3S)-isocitrate. Does –OH add from the Re face of the double bond or from the Si face? What about –H? Does the addition of water occur with syn or anti geometry?
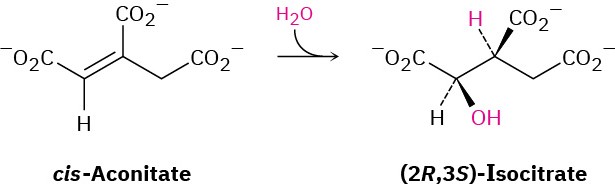
General Problems
Problem 29-51
In glycerol metabolism, the oxidation of sn-glycerol 3-phosphate to give dihydroxyacetone phosphate is catalyzed by sn-glycerol-3-phosphate dehydrogenase, with NAD+ as cofactor. The reaction is stereospecific, occurring exclusively on the Re face of the nicotinamide ring.
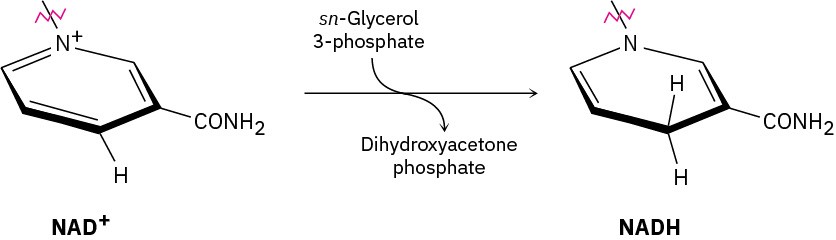
Which hydrogen in the NADH product comes from sn-glycerol 3-phosphate? Does it have pro-R or pro-S stereochemistry?
Problem 29-52
The primary fate of acetyl CoA under normal metabolic conditions is degradation in the citric acid cycle to yield CO2. When the body is stressed by prolonged starvation, however, acetyl CoA is converted into compounds called ketone bodies, which can be used by the brain as a temporary fuel. Fill in the missing information indicated by the four question marks in the following biochemical pathway for the synthesis of ketone bodies from acetyl CoA:
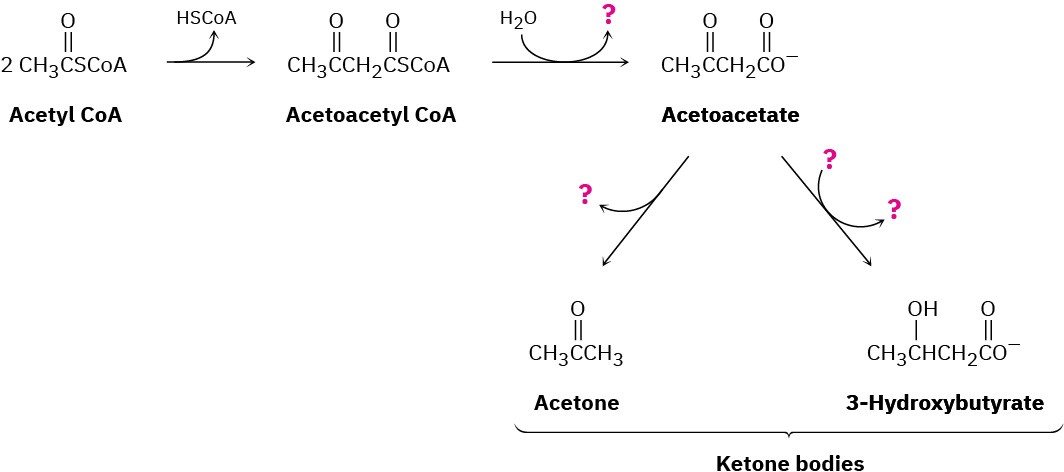
Problem 29-53
The initial reaction in Problem 29-52, conversion of two molecules of acetyl CoA to one molecule of acetoacetyl CoA, is a Claisen reaction. Assuming that there is a base present, show the mechanism of the reaction.
Problem 29-54
In step 6 of fatty-acid biosynthesis (Figure 29.6), acetoacetyl ACP is reduced stereospecifically by NADPH to yield an alcohol. Does hydride ion add to the Si face or the Re face of acetoacetyl ACP?
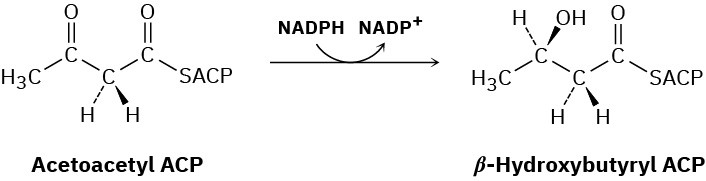
Problem 29-55
In step 7 of fatty-acid biosynthesis (Figure 29.6), dehydration of a β-hydroxy thioester occurs to give trans-crotonyl ACP. Is the dehydration a syn elimination or an anti elimination?
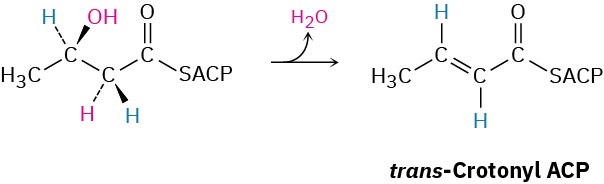
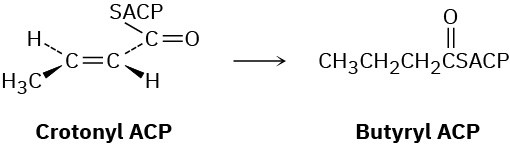
Problem 29-56
In step 8 of fatty-acid biosynthesis (Figure 29.6), reduction of trans-crotonyl ACP gives butyryl ACP. A hydride from NADPH adds to C3 of the crotonyl group from the Re face, and protonation on C2 occurs on the Si face. Is the reduction a syn addition or an anti addition?