- Absolute configuration (Section 5.5): The exact three-dimensional structure of a chiral molecule. Absolute configurations are specified verbally by the Cahn–Ingold– Prelog R,S convention.
- Absorbance (A) (Section 14.7): In optical spectroscopy, the logarithm of the intensity of the incident light divided by the intensity of the light transmitted through a sample; A = log I0/I.
- Absorption spectrum (Section 12.5): A plot of wavelength of incident light versus amount of light absorbed. Organic molecules show absorption spectra in both the infrared and the ultraviolet regions of the electromagnetic spectrum.
- Acetals, R2C(OR′)2 (Section 19.10): A type of functional group consisting of two −OR groups bonded to the same carbon, R2C(OR′)2. Acetals are often used as protecting groups for ketones and aldehydes.
- Acetoacetic ester synthesis (Section 22.7): The synthesis of a methyl ketone by alkylation of an alkyl halide with ethyl acetoacetate, followed by hydrolysis and decarboxylation.
- Acetyl group (Section 19.1): The CH3CO− group.
- Acetylide anion (Section 9.7): The anion formed by removal of a proton from a terminal alkyne, R–C≡C:!.
- Achiral (Section 5.2): Having a lack of handedness. A molecule is achiral if it has a plane of symmetry and is thus superimposable on its mirror image.
- Acid anhydrides (Chapter 21 Introduction): A type of functional group with two acyl groups bonded to a common oxygen atom, RCO2COR′.
- Acid halides (Chapter 21 Introduction): A type of functional group with an acyl group bonded to a halogen atom, RCOX.
- Acidity constant, Ka (Section 2.8): A measure of acid strength. For any acid HA, the acidity constant is given by the expression 𝐾a [H!O”][A#]. [HA]
- Activating groups (Section 16.4): Electron-donating groups such as hydroxyl (−OH) or amino (−NH2) that increase the reactivity of an aromatic ring toward electrophilic aromatic substitution.
- Activation energy (Section 6.9): The difference in energy between ground state and transition state in a reaction. The amount of activation energy determines the rate at which the reaction proceeds. Most organic reactions have activation energies of 40– 100 kJ/mol.
- Active site (Section 6.11, Section 26.11): The pocket in an enzyme where a substrate is bound and undergoes reaction.
- Acyclic diene metathesis (ADMET) (Section 31.5): A method of polymer synthesis that uses the olefin metathesis reaction of an open-chain diene.
- Acyl group (Section 16.3, Section 19.1): A −COR group.
- Acyl phosphates (Chapter 21 Introduction): A type of functional group with an acyl group bonded to a phosphate, RCO2PO32−.
- Acylation (Section 16.3, Section 24.7): The introduction of an acyl group, −COR, onto a molecule. For example, acylation of an alcohol yields an ester, acylation of an amine yields an amide, and acylation of an aromatic ring yields an alkyl aryl ketone.
- Acylium ion (Section 16.3): A resonance-stabilized carbocation in which the positive charge is located at a carbonyl-group carbon, R–C═O ↔ R–C≡O ions are intermediates in Friedel–Crafts acylation reactions.
- Adams’ catalyst (Section 6.11): The PtO2 catalyst used for alkene hydrogenations.
- 1,2-Addition (Section 14.2, Section 19.13): Addition of a reactant to the two ends of a double bond.
- 1,4-Addition (Section 14.2, Section 19.13): Addition of a reactant to the ends of a conjugated π system. Conjugated dienes yield 1,4 adducts when treated with electrophiles such as HCl. Conjugated enones yield 1,4 adducts when treated with nucleophiles such as amines.
- Addition reactions (Section 6.1): Occur when two reactants add together to form a single product with no atoms left over.
- Adrenocortical hormones (Section 27.6): Steroid hormones secreted by the adrenal glands. There are two types of these hormones: mineralocorticoids and glucocorticoids.
- Alcohols (Section 3.1, Chapter 17 Introduction): A class of compounds with an −OH group bonded to a saturated, sp3-hybridized carbon, ROH.
- Aldaric acid (Section 25.6): The dicarboxylic acid resulting from oxidation of an aldose.
- Aldehydes (RCHO) (Section 3.1, Chapter 19 Introduction): A class of compounds containing the −CHO functional group.
- Alditol (Section 25.6): The polyalcohol resulting from reduction of the carbonyl group of a sugar.
- Aldol reaction (Section 23.1): The carbonyl condensation reaction of an aldehyde or ketone to give a β-hydroxy carbonyl compound.
- Aldonic acids (Section 25.6): Monocarboxylic acids resulting from oxidation of the −CHO group of an aldose.
- Aldoses (Section 25.1): A type of carbohydrate with an aldehyde functional group.
- Alicyclic (Section 4.1): A nonaromatic cyclic hydrocarbon such as a cycloalkane or cycloalkene.
- Aliphatic (Section 3.2): A nonaromatic hydrocarbon such as a simple alkane, alkene, or alkyne.
- Alkaloids (Chapter 2 Chemistry Matters): Naturally occurring organic bases, such as morphine.
- Alkanes (Section 3.2): A class of compounds of carbon and hydrogen that contains only single bonds.
- Alkene (Section 3.1, Chapter 7 Introduction): A hydrocarbon that contains a carbon–carbon double bond, R%C═CR%.
- Alkoxide ion, RO− (Section 17.2): The anion formed by deprotonation of an alcohol.
- Alkoxymercuration (Section 18.2): A method for synthesizing ethers by mercuric- ion catalyzed addition of an alcohol to an alkene followed by demercuration on treatment with NaBH4.
- Alkyl group (Section 3.3): The partial structure that remains when a hydrogen atom is removed from an alkane.
- Alkyl halide (Section 3.1, Chapter 10 Introduction): A compound with a halogen atom bonded to a saturated, sp3-hybridized carbon atom.
- Alkylamines (Section 24.1): Amino-substituted alkanes RNH2, R2NH, or R3N.
- Alkylation (Section 9.8, Section 16.3, Section 18.2, Section 22.7): Introduction of an alkyl group onto a molecule. For example, aromatic rings can be alkylated to yield arenes, and enolate anions can be alkylated to yield α-substituted carbonyl compounds.
- Alkyne (Section 3.1, Chapter 9 Introduction): A hydrocarbon that contains a carbon–carbon triple bond, RC≡CR.
- Allyl group (Section 7.3): A H%C═CHCH% − substituent.
- Allylic (Section 10.3): The position next to a double bond. For example, H%C═CHCH%Br is an allylic bromide.
- α-Amino acids (Section 26.1): A type of difunctional compound with an amino group on the carbon atom next to a carboxyl group, RCH(NH2)CO2H.
- α Anomer (Section 25.5): The cyclic hemiacetal form of a sugar that has the hemiacetal −OH group cis to the −OH at the lowest chirality center in a Fischer projection.
- α Helix (Section 26.9): The coiled secondary structure of a protein.
- α Position (Chapter 22 Introduction): The position next to a carbonyl group.
- α-Substitution reaction (Chapter 22 Introduction): The substitution of the α hydrogen atom of a carbonyl compound by reaction with an electrophile.
- Amides (Section 3.1, Chapter 21 Introduction): A class of compounds containing the −CONR2 functional group.
- Amidomalonate synthesis (Section 26.3): A method for preparing α-amino acids by alkylation of diethyl amidomalonate with an alkyl halide followed by deprotection and decarboxylation.
- Amines (Section 3.1, Chapter 24 Introduction): A class of compounds containing one or more organic substituents bonded to a nitrogen atom, RNH2, R2NH, or R3N.
- Amino acid (Section 26.1): See α-Amino acid.
- Amino sugar (Section 25.7): A sugar with one of its −OH groups replaced by −NH2.
- Amphiprotic (Section 26.1): Capable of acting either as an acid or as a base. Amino acids are amphiprotic.
- Amplitude (Section 12.5): The height of a wave measured from the midpoint to the maximum. The intensity of radiant energy is proportional to the square of the wave’s amplitude.
- Anabolic steroids (Section 27.6): Synthetic androgens that mimic the tissue- building effects of natural testosterone.
- Anabolism (Section 29.1): The group of metabolic pathways that build up larger molecules from smaller ones.
- Androgen (Section 27.6): A male steroid sex hormone.
- Angle strain (Section 4.3): The strain introduced into a molecule when a bond angle is deformed from its ideal value. Angle strain is particularly important in small-ring cycloalkanes, where it results from compression of bond angles to less than their ideal tetrahedral values.
- Annulation (Section 23.12): The building of a new ring onto an existing molecule.
- Anomeric center (Section 25.5): The hemiacetal carbon atom in the cyclic pyranose or furanose form of a sugar.
- Anomers (Section 25.5): Cyclic stereoisomers of sugars that differ only in their configuration at the hemiacetal (anomeric) carbon.
- Antarafacial (Section 30.5): A pericyclic reaction that takes place on opposite faces of the two ends of a π electron system.
- Anti conformation (Section 3.7): The geometric arrangement around a carbon– carbon single bond in which the two largest substituents are 180° apart as viewed in a Newman projection.
- Anti periplanar (Section 11.8): Describing the stereochemical relationship in which two bonds on adjacent carbons lie in the same plane at an angle of 180°.
- Anti stereochemistry (Section 8.2): The opposite of syn. An anti addition reaction is one in which the two ends of the double bond are attacked from different sides. An anti elimination reaction is one in which the two groups leave from opposite sides of the molecule.
- Antiaromatic (Section 15.3): Referring to a planar, conjugated molecule with 4n π electrons. Delocalization of the π electrons leads to an increase in energy.
- Antibonding MO (Section 1.11): A molecular orbital that is higher in energy than the atomic orbitals from which it is formed.
- Anticodon (Section 28.5): A sequence of three bases on tRNA that reads the codons on mRNA and brings the correct amino acids into position for protein synthesis.
- Antisense strand (Section 28.4): The template, noncoding strand of double-helical DNA that does not contain the gene.
- Arene (Section 15.1): An alkyl-substituted benzene.$
- Arenediazonium salt (Section 24.8): An aromatic compound Ar–N≡N X!; used in the Sandmeyer reaction.
- Aromaticity (Chapter 15 Introduction): The special characteristics of cyclic conjugated molecules, including unusual stability and a tendency to undergo substitution reactions rather than addition reactions on treatment with electrophiles. Aromatic molecules are planar, cyclic, conjugated species with 4n + 2 π electrons.
- Arylamines (Section 24.1): Amino-substituted aromatic compounds, ArNH2.
- Atactic (Section 31.2): A chain-growth polymer in which the stereochemistry of the substituents is oriented randomly along the backbone.
- Atomic mass (Section 1.1): The weighted average mass of an element’s naturally occurring isotopes.
- Atomic number, Z (Section 1.1): The number of protons in the nucleus of an atom.
- ATZ Derivative (Section 26.6): An anilinothiazolinone, formed from an amino acid during Edman degradation of a peptide.
- Aufbau principle (Section 1.3): The rules for determining the electron configuration of an atom.
- Axial bonds (Section 4.6): Bonds or positions in chair cyclohexane that lie along the ring axis, perpendicular to the rough plane of the ring.
- Azide synthesis (Section 24.6): A method for preparing amines by SN2 reaction of an alkyl halide with azide ion, followed by reduction.
- Azo compounds (Section 24.8): A class of compounds with the general structure R–N═N–R’.
- Backbone (Section 26.4): The continuous chain of atoms running the length of a protein or other polymer.
- Base peak (Section 12.1): The most intense peak in a mass spectrum.
- Basicity constant, Kb (Section 24.3): A measure of base strength in water. For any base B, the basicity constant is given by the expression
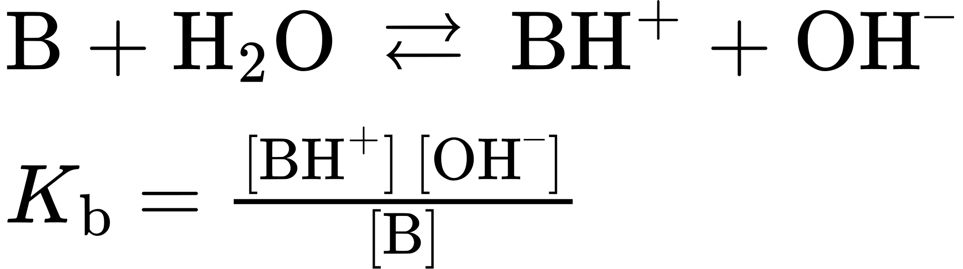
- Bent bonds (Section 4.4): The bonds in small rings such as cyclopropane that bend away from the internuclear line and overlap at a slight angle, rather than head-on. Bent bonds are highly strained and highly reactive.
- Benzoyl (Section 19.1): The C6H5CO− group.
- Benzyl (Section 15.1): The C6H5CH2− group.
- Benzylic (Section 11.5): The position next to an aromatic ring.
- Benzyne (Section 16.7): An unstable compound having a triple bond in a benzene ring.
- β Anomer (Section 25.5): The cyclic hemiacetal form of a sugar that has the hemiacetal −OH group trans to the −OH at the lowest chirality center in a Fischer projection.
- β Diketone (Section 22.5): A 1,3-diketone.
- β-Keto ester (Section 22.5): A 3-oxoester.
- β Lactam (Chapter 21 Chemistry Matters): A four-membered lactam, or cyclic amide. Penicillin and cephalosporin antibiotics contain β-lactam rings.
- β-Oxidation pathway (Section 29.3): The metabolic pathway for degrading fatty acids.
- β-Pleated sheet (Section 26.9): A type of secondary structure of a protein.
- Betaine (Section 19.11): A neutral dipolar molecule with nonadjacent positive and negative charges. For example, the adduct of a Wittig reagent with a carbonyl compound is a betaine.
- Bicycloalkane (Section 4.9): A cycloalkane that contains two rings.
- Bimolecular reaction (Section 11.2): A reaction whose rate-limiting step occurs between two reactants.
- Block copolymers (Section 31.3): Polymers in which different blocks of identical monomer units alternate with one another.
- Boat cyclohexane (Section 4.5): A conformation of cyclohexane that bears a slight resemblance to a boat. Boat cyclohexane has no angle strain but has a large number of eclipsing interactions that make it less stable than chair cyclohexane.
- Boc derivative (Section 26.7): A butyloxycarbonyl N-protected amino acid.
- Bond angle (Section 1.6): The angle formed between two adjacent bonds.
- Bond dissociation energy, D (Section 6.8): The amount of energy needed to break a bond and produce two radical fragments.
- Bond length (Section 1.5): The equilibrium distance between the nuclei of two atoms that are bonded to each other.
- Bond strength (Section 1.5): An alternative name for bond dissociation energy.
- Bonding MO (Section 1.11): A molecular orbital that is lower in energy than the atomic orbitals from which it is formed.
- Branched-chain alkanes (Section 3.2): Alkanes that contain a branching connection of carbons as opposed to straight-chain alkanes.
- Bridgehead (Section 4.9): An atom that is shared by more than one ring in a polycyclic molecule.
- Bromohydrin (Section 8.3): A 1,2-bromoalcohol; obtained by addition of HOBr to an alkene.
- Bromonium ion (Section 8.2): A species with a divalent, positively charged bromine, R2Br+.
- Brønsted–Lowry acid (Section 2.7): A substance that donates a hydrogen ion (proton; H+) to a base.
- Brønsted–Lowry base (Section 2.7): A substance that accepts H+ from an acid.
- C-terminal amino acid (Section 26.4): The amino acid with a free −CO2H group at the end of a protein chain.
- Cahn–Ingold–Prelog sequence rules (Section 5.5, Section 7.5): A series of rules for assigning relative rankings to substituent groups on a chirality center or a double- bond carbon atom.
- Cannizzaro reaction (Section 19.12): The disproportionation reaction of an aldehyde on treatment with base to yield an alcohol and a carboxylic acid.
- Carbanion (Section 10.6, Section 19.7): A carbon anion, or substance that contains a trivalent, negatively charged carbon atom (R3C:−). Alkyl carbanions are sp3– hybridized and have eight electrons in the outer shell of the negatively charged carbon.
- Carbene, R2C (Section 8.9): A neutral substance that contains a divalent carbon atom having only six electrons in its outer shell (R2C:).
- Carbinolamine (Section 19.8): A molecule that contains the R2C(OH)NH2 functional group. Carbinolamines are produced as intermediates during the nucleophilic addition of amines to carbonyl compounds.
- Carbocation (Section 6.4, Section 7.9): A carbon cation, or substance that contains a trivalent, positively charged carbon atom having six electrons in its outer shell (R3C+).
- Carbohydrates (Chapter 25 Introduction): Polyhydroxy aldehydes or ketones. Carbohydrates can be either simple sugars, such as glucose, or complex sugars, such as cellulose.
- Carbonyl condensation reactions (Section 23.1): A type of reaction that joins two carbonyl compounds together by a combination of α-substitution and nucleophilic addition reactions.
- Carbonyl group (Section 3.1, Preview of Carbonyl Chemistry): The C═O functional group.
- Carboxyl group (Section 20.1): The −CO2H functional group.
- Carboxylation (Section 20.5): The addition of CO2 to a molecule.
- Carboxylic acids, RCO2H (Section 3.1, Chapter 20 Introduction): Compounds containing the −CO2H functional group.
- Carboxylic acid derivative (Chapter 21 Introduction): A compound in which an acyl group is bonded to an electronegative atom or substituent that can act as a leaving group in a substitution reaction. Esters, amides, and acid halides are examples.
- Catabolism (Section 29.1): The group of metabolic pathways that break down larger molecules into smaller ones.
- Catalyst (Section 6.11): A substance that increases the rate of a chemical transformation by providing an alternative mechanism but is not itself changed in the reaction.
- Cation radical (Section 12.1): A reactive species, typically formed in a mass spectrometer by loss of an electron from a neutral molecule and having both a positive charge and an odd number of electrons.
- Chain-growth polymers (Section 8.10, Section 21.9, Section 31.1): Polymers whose bonds are produced by chain reaction mechanisms. Polyethylene and other alkene polymers are examples.
- Chain reaction (Section 6.6): A reaction that, once initiated, sustains itself in an endlessly repeating cycle of propagation steps. The radical chlorination of alkanes is an example of a chain reaction that is initiated by irradiation with light and then continues in a series of propagation steps.
- Chair conformation (Section 4.5): A three-dimensional conformation of cyclohexane that resembles the rough shape of a chair. The chair form of cyclohexane is the lowest-energy conformation of the molecule.
- Chemical shift (Section 13.3): The position on the NMR chart where a nucleus absorbs. By convention, the chemical shift of tetramethylsilane (TMS) is set at zero, and all other absorptions usually occur downfield (to the left on the chart). Chemical shifts are expressed in delta units (δ), where 1 δ equals 1 ppm of the spectrometer operating frequency.
- Chiral (Section 5.2): Having handedness. Chiral molecules are those that do not have a plane of symmetry and are therefore not superimposable on their mirror image. A chiral molecule thus exists in two forms, one right-handed and one left- handed. The most common cause of chirality in a molecule is the presence of a carbon atom that is bonded to four different substituents.
- Chiral environment (Section 5.12): The chiral surroundings or conditions in which a molecule resides.
- Chirality center (Section 5.2): An atom (usually carbon) that is bonded to four different groups.
- Chlorohydrin (Section 8.3): A 1,2-chloroalcohol; obtained by addition of HOCl to an alkene.
- Chromatography (Section 26.5): A technique for separating a mixture of compounds into pure components. Different compounds adsorb to a stationary support phase and are then carried along it at different rates by a mobile phase.
- Cis–trans isomers (Section 4.2, Section 7.4): Stereoisomers that differ in their stereochemistry about a ring or double bond.
- Citric acid cycle (Section 29.7): The metabolic pathway by which acetyl CoA is degraded to CO2.
- Claisen condensation reaction (Section 23.7): The carbonyl condensation reaction of two ester molecules to give a β-keto ester product.
- Claisen rearrangement (Section 30.8): The pericyclic conversion of an allyl phenyl ether to an o-allylphenol or an allyl vinyl ether to a γ,δ-unsaturated ketone by heating.
- Coding strand (Section 28.4): The sense strand of double-helical DNA that contains the gene.
- Codon (Section 28.5): A three-base sequence on a messenger RNA chain that encodes the genetic information necessary to cause a specific amino acid to be incorporated into a protein. Codons on mRNA are read by complementary anticodons on tRNA.
- Coenzyme (Section 26.10): A small organic molecule that acts as a cofactor in a biological reaction.
- Cofactor (Section 26.10): A small nonprotein part of an enzyme that is necessary for biological activity.
- Combinatorial chemistry (Chapter 16 Chemistry Matters): A procedure in which anywhere from a few dozen to several hundred thousand substances are prepared simultaneously.
- Complex carbohydrates (Section 25.1): Carbohydrates that are made of two or more simple sugars linked together by glycoside bonds.
- Concerted reaction (Chapter 30 Introduction): A reaction that takes place in a single step without intermediates. For example, the Diels–Alder cycloaddition reaction is a concerted process.
- Condensed structures (Section 1.12): A shorthand way of writing structures in which carbon–hydrogen and carbon–carbon bonds are understood rather than shown explicitly. Propane, for example, has the condensed structure CH3CH2CH3.
- Configuration (Section 5.5): The three-dimensional arrangement of atoms bonded to a chirality center.
- Conformations (Section 3.6): The three-dimensional shape of a molecule at any given instant, assuming that rotation around single bonds is frozen.
- Conformational analysis (Section 4.8): A means of assessing the energy of a substituted cycloalkane by totaling the steric interactions present in the molecule.
- Conformers (Section 3.6): Conformational isomers.
- Conjugate acid (Section 2.7): The product that results from protonation of a Brønsted–Lowry base.
- Conjugate addition (Section 19.13): Addition of a nucleophile to the β carbon atom of an α,β-unsaturated carbonyl compound.
- Conjugate base (Section 2.7): The product that results from deprotonation of a Brønsted–Lowry acid.
- Conjugation (Chapter 14 Introduction): A series of overlapping p orbitals, usually in alternating single and multiple bonds. For example, 1,3-butadiene is a conjugated diene, 3-buten-2-one is a conjugated enone, and benzene is a cyclic conjugated triene.
- Conrotatory (Section 30.2): A term used to indicate that p orbitals must rotate in the same direction during electrocyclic ring-opening or ring-closure.
- Constitutional isomers (Section 3.2, Section 3.6, Section 5.9): Isomers that have their atoms connected in a different order. For example, butane and 2- methylpropane are constitutional isomers.
- Cope rearrangement (Section 30.8): The sigmatropic rearrangement of a 1,5- hexadiene.
- Copolymers (Section 31.3): Polymers obtained when two or more different monomers are allowed to polymerize together.
- Coupled reactions (Section 29.1): Two reactions that share a common intermediate so that the energy released in the favorable step allows the unfavorable step to occur.
- Coupling constant, J (Section 13.6): The magnitude (expressed in hertz) of the interaction between nuclei whose spins are coupled.
- Covalent bond (Section 1.4, Section 1.5): A bond formed by sharing electrons between atoms.
- Cracking (Chapter 3 Chemistry Matters): A process used in petroleum refining in which large alkanes are thermally cracked into smaller fragments.
- Crown ethers (Section 18.6): Large-ring polyethers; used as phase-transfer catalysts.
- Crystallites (Section 31.7): Highly ordered crystal-like regions within a long polymer chain.
- Curtius rearrangement (Section 24.6): The conversion of an acid chloride into an amine by reaction with azide ion, followed by heating with water.
- Cyanohydrins (Section 19.6): A class of compounds with an −OH group and a −CN group bonded to the same carbon atom; formed by addition of HCN to an aldehyde or ketone.
- Cycloaddition reaction (Section 14.4, Section 30.5): A pericyclic reaction in which two reactants add together in a single step to yield a cyclic product. The Diels–Alder reaction between a diene and a dienophile to give a cyclohexene is an example.
- Cycloalkane (Section 4.1): An alkane that contains a ring of carbons.
- D Sugars (Section 25.3): Sugars whose hydroxyl group at the chirality center farthest from the carbonyl group has the same configuration as D-glyceraldehyde and points to the right when drawn in Fischer projection.
- d,l form (Section 5.8): The racemic mixture of a chiral compound.
- Deactivating groups (Section 16.4): Electron-withdrawing substituents that decrease the reactivity of an aromatic ring toward electrophilic aromatic substitution.
- Deamination (Section 29.9): The removal of an amino group from a molecule, as occurs with amino acids during metabolic degradation.
- Debyes (D) (Section 2.2): Units for measuring dipole moments; 1 D = 3.336 × 10−30 coulomb meter (C ∙ m).
- Decarboxylation (Section 22.7): The loss of carbon dioxide from a molecule. β-Keto acids decarboxylate readily on heating.
- Degenerate orbitals (Section 15.2): Two or more orbitals that have the same energy level.
- Degree of unsaturation (Section 7.2): The number of rings and/or multiple bonds in a molecule.
- Dehydration (Section 8.1, Section 12.3, Section 17.6): The loss of water from an alcohol to yield an alkene.
- Dehydrohalogenation (Section 8.1, Section 9.2): The loss of HX from an alkyl halide. Alkyl halides undergo dehydrohalogenation to yield alkenes on treatment with strong base.
- Delocalization (Section 10.4, Section 15.2): A spreading out of electron density over a conjugated π electron system. For example, allylic cations and allylic anions are delocalized because their charges are spread out over the entire π electron system. Aromatic compounds have 4n + 2 π electrons delocalized over their ring.
- Delta (δ) scale (Section 13.3): An arbitrary scale used to calibrate NMR charts. One delta unit (δ) is equal to 1 part per million (ppm) of the spectrometer operating frequency.
- Denatured (Section 26.9): The physical changes that occur in a protein when secondary and tertiary structures are disrupted.
- Deoxy sugar (Section 25.7): A sugar with one of its −OH groups replaced by an −H.
- Deoxyribonucleic acid (DNA) (Chapter 28 Introduction): The biopolymer consisting of deoxyribonucleotide units linked together through phosphate–sugar bonds. Found in the nucleus of cells, DNA contains an organism’s genetic information.
- DEPT-NMR (Section 13.12): An NMR method for distinguishing among signals due to CH3, CH2, CH, and quaternary carbons. That is, the number of hydrogens attached to each carbon can be determined.
- Deshielding (Section 13.2): An effect observed in NMR that causes a nucleus to absorb toward the left (downfield) side of the chart. Deshielding is caused by a withdrawal of electron density from the nucleus.
- Dess–Martin periodinane (Section 17.7): An iodine-based reagent commonly used for the laboratory oxidation of a primary alcohol to an aldehyde or a secondary alcohol to a ketone.
- Deuterium isotope effect (Section 11.8): A tool used in mechanistic investigations to establish whether a C−H bond is broken in the rate-limiting step of a reaction.
- Dextrorotatory (Section 5.3): A word used to describe an optically active substance that rotates the plane of polarization of plane-polarized light in a right-handed (clockwise) direction.
- Diastereomers (Section 5.6): Non–mirror-image stereoisomers; diastereomers have the same configuration at one or more chirality centers but differ at other chirality centers.
- Diastereotopic (Section 13.7): Hydrogens in a molecule whose replacement by some other group leads to different diastereomers.
- 1,3-Diaxial interaction (Section 4.7): The strain energy caused by a steric interaction between axial groups three carbon atoms apart in chair cyclohexane.
- Diazonium salts (Section 24.8): A type of compound with the general structure RN2+ X−.
- Diazotization (Section 24.8): The conversion of a primary amine, RNH2, into a diazonium ion, RN2+, by treatment with nitrous acid.
- Dieckmann cyclization reaction (Section 23.9): An intramolecular Claisen condensation reaction of a diester to give a cyclic β-keto ester.
- Diels–Alder reaction (Section 14.4, Section 30.5): The cycloaddition reaction of a diene with a dienophile to yield a cyclohexene.
- Dienophile (Section 14.5): A compound containing a double bond that can take part in the Diels–Alder cycloaddition reaction. The most reactive dienophiles are those that have electron-withdrawing groups on the double bond.
- Digestion (Section 29.1): The first stage of catabolism, in which food is broken down by hydrolysis of ester, glycoside (acetal), and peptide (amide) bonds to yield fatty acids, simple sugars, and amino acids.
- Dihedral angle (Section 3.6): The angle between two bonds on adjacent carbons as viewed along the C−C bond.
- Dipole moment, µ (Section 2.2): A measure of the net polarity of a molecule. A dipole moment arises when the centers of mass of positive and negative charges within a molecule do not coincide.
- Dipole–dipole forces (Section 2.12): Noncovalent electrostatic interactions between dipolar molecules.
- Disaccharide (Section 25.8): A carbohydrate formed by linking two simple sugars through an acetal bond.
- Dispersion forces (Section 2.12): Noncovalent interactions between molecules that arise because of constantly changing electron distributions within the molecules.
- Disrotatory (Section 30.2): A term used to indicate that p orbitals rotate in opposite directions during electrocyclic ring-opening or ring-closing reactions.
- Disulfides (RSSR′) (Section 18.7): A class of compounds of the general structure RSSR′.
- Deoxyribonucleic acid (DNA) (Chapter 28 Introduction): Chemical carriers of a cell’s genetic information.
- Double bond (Section 1.8): A covalent bond formed by sharing two electron pairs between atoms.
- Double helix (Section 28.2): The structure of DNA in which two polynucleotide strands coil around each other.
- Doublet (Section 13.6): A two-line NMR absorption caused by spin–spin splitting when the spin of the nucleus under observation couples with the spin of a neighboring magnetic nucleus.
- Downfield (Section 13.3): Referring to the left-hand portion of the NMR chart.
- E geometry (Section 7.5): A term used to describe the stereochemistry of a carbon– carbon double bond. The two groups on each carbon are ranked according to the Cahn–Ingold–Prelog sequence rules, and the two carbons are compared. If the higher-ranked groups on each carbon are on opposite sides of the double bond, the bond has E geometry.
- E1 reaction (Section 11.10): A unimolecular elimination reaction in which the substrate spontaneously dissociates to give a carbocation intermediate, which loses a proton in a separate step.
- E1cB reaction (Section 11.10): A unimolecular elimination reaction in which a proton is first removed to give a carbanion intermediate, which then expels the leaving group in a separate step.
- E2 reaction (Section 11.8): A bimolecular elimination reaction in which C−H and C−X bond cleavages are simultaneous.
- Eclipsed conformation (Section 3.6): The geometric arrangement around a carbon–carbon single bond in which the bonds to substituents on one carbon are parallel to the bonds to substituents on the neighboring carbon as viewed in a Newman projection.
- Eclipsing strain (Section 3.6): The strain energy in a molecule caused by electron repulsions between eclipsed bonds. Eclipsing strain is also called torsional strain.
- Edman degradation (Section 26.6): A method for N-terminal sequencing of peptide chains by treatment with N-phenylisothiocyanate.
- Eicosanoid (Section 27.4): A lipid derived biologically from 5,8,11,14- eicosatetraenoic acid, or arachidonic acid. Prostaglandins, thromboxanes, and leukotrienes are examples.
- Elastomer (Section 31.7): An amorphous polymer that has the ability to stretch out and spring back to its original shape.
- Electrocyclic reaction (Section 30.2): A unimolecular pericyclic reaction in which a ring is formed or broken by a concerted reorganization of electrons through a cyclic transition state. For example, the cyclization of 1,3,5-hexatriene to yield 1,3- cyclohexadiene is an electrocyclic reaction.
- Electromagnetic spectrum (Section 12.5): The range of electromagnetic energy, including infrared, ultraviolet, and visible radiation.
- Electron configuration (Section 1.3): A list of the orbitals occupied by electrons in an atom.
- Electron-dot structure (Section 1.4): A representation of a molecule showing valence electrons as dots.
- Electron shells (Section 1.2): A group of an atom’s electrons with the same principal quantum number.
- Electron-transport chain (Section 29.1): The final stage of catabolism in which ATP is produced.
- Electronegativity (EN) (Section 2.1): The ability of an atom to attract electrons in a covalent bond. Electronegativity increases across the periodic table from left to right and from bottom to top.
- Electrophile (Section 6.3, Section 6.4): An “electron-lover,” or substance that accepts an electron pair from a nucleophile in a polar bond-forming reaction.
- Electrophilic addition reactions (Section 7.7): Addition of an electrophile to a carbon–carbon double bond to yield a saturated product.
- Electrophilic aromatic substitution reaction (Chapter 16 Introduction): A reaction in which an electrophile (E+) reacts with an aromatic ring and substitutes for one of the ring hydrogens.
- Electrophoresis (Section 26.2, Section 28.6): A technique used for separating charged organic molecules, particularly proteins and DNA fragments. The mixture to be separated is placed on a buffered gel or paper, and an electric potential is applied across the ends of the apparatus. Negatively charged molecules migrate toward the positive electrode, and positively charged molecules migrate toward the negative electrode.
- Electrostatic potential maps (Section 2.1): Molecular representations that use color to indicate the charge distribution in molecules as derived from quantum- mechanical calculations.
- Elimination reactions (Section 6.1): What occurs when a single reactant splits into two products.
- Elution (Section 26.5): The passage of a substance from a chromatography column.
- Embden–Meyerhof pathway (Section 29.5): An alternative name for glycolysis.
- Enamines (Section 19.8): Compounds with the R%N–CR═CR% functional group.
- Enantiomers (Section 5.1): Stereoisomers of a chiral substance that have a mirror- image relationship. Enantiomers have opposite configurations at all chirality centers.
- Enantioselective synthesis (Chapter 19 Chemistry Matters): A reaction method that yields only a single enantiomer of a chiral product starting from an achiral reactant.
- Enantiotopic (Section 13.7): Hydrogens in a molecule whose replacement by some other group leads to different enantiomers.
- 3′ End (Section 28.1): The end of a nucleic acid chain with a free hydroxyl group at C3′.
- 5′ End (Section 28.1): The end of a nucleic acid chain with a free hydroxyl group at C5′.
- Endergonic (Section 6.7): A reaction that has a positive free-energy change and is therefore nonspontaneous. In an energy diagram, the product of an endergonic reaction has a higher energy level than the reactants.
- Endo (Section 14.5): A term indicating the stereochemistry of a substituent in a bridged bicycloalkane. An endo substituent is syn to the larger of the two bridges.
- Endothermic (Section 6.7): A reaction that absorbs heat and therefore has a positive enthalpy change.
- Energy diagram (Section 6.9): A representation of the course of a reaction, in which free energy is plotted as a function of reaction progress. Reactants, transition states, intermediates, and products are represented, and their appropriate energy levels are indicated.
- Enol (Section 9.4, Section 22.1): A vinylic alcohol that is in equilibrium with a carbonyl compound, C═C–OH.
- Enolate ion (Section 22.1): The anion of an enol, C═C–O!.
- Enthalpy change (ΔH) (Section 6.7): The heat of reaction. The enthalpy change that occurs during a reaction is a measure of the difference in total bond energy between reactants and products.
- Entropy change (ΔS) (Section 6.7): The change in amount of molecular randomness. The entropy change that occurs during a reaction is a measure of the difference in randomness between reactants and products.
- Enzyme (Section 6.11, Section 26.10): A biological catalyst. Enzymes are large proteins that catalyze specific biochemical reactions.
- Epimers (Section 5.6): Diastereomers that differ in configuration at only one chirality center but are the same at all others.
- Epoxide (Section 8.7): A three-membered-ring ether functional group.
- Equatorial bonds (Section 4.6): Bonds or positions in chair cyclohexane that lie along the rough equator of the ring.
- ESI (Section 12.4): Electrospray ionization; a “soft” ionization method used for mass spectrometry of biological samples of very high molecular weight.
- Essential amino acid (Section 26.1): One of nine amino acids that are biosynthesized only in plants and microorganisms and must be obtained by humans in the diet.
- Essential monosaccharide (Section 25.7): One of eight simple sugars that is best obtained in the diet rather than by biosynthesis.
- Essential oil (Chapter 8 Chemistry Matters): The volatile oil obtained by steam distillation of a plant extract.
- Esters (Section 3.1, Chapter 21 Introduction): A class of compounds containing the −CO2R functional group.
- Estrogens (Section 27.6): Female steroid sex hormones.
- Ethers (Section 3.1, Chapter 18 Introduction): A class of compounds that has two organic substituents bonded to the same oxygen atom, ROR′.
- Exergonic (Section 6.7): A reaction that has a negative free-energy change and is therefore spontaneous. On an energy diagram, the product of an exergonic reaction has a lower energy level than that of the reactants.
- Exo (Section 14.5): A term indicating the stereochemistry of a substituent in a bridged bicycloalkane. An exo substituent is anti to the larger of the two bridges.
- Exon (Section 28.4): A section of DNA that contains genetic information.
- Exothermic (Section 6.7): A reaction that releases heat and therefore has a negative enthalpy change.
- Fats (Section 27.1): Solid triacylglycerols derived from an animal source.
- Fatty acids (Section 27.1): A long, straight-chain carboxylic acid found in fats and oils.
- Fiber (Section 31.7): A thin thread produced by extruding a molten polymer through small holes in a die.
- Fibrous proteins (Section 26.9): A type of protein that consists of polypeptide chains arranged side by side in long threads. Such proteins are tough, insoluble in water, and used in nature for structural materials such as hair, hooves, and fingernails.
- Fingerprint region (Section 12.7): The complex region of the infrared spectrum from 1500–400 cm−1.
- First-order reaction (Section 11.4): Designates a reaction whose rate-limiting step is unimolecular and whose kinetics therefore depend on the concentration of only one reactant.
- Fischer esterification reaction (Section 21.3): The acid-catalyzed nucleophilic acyl substitution reaction of a carboxylic acid with an alcohol to yield an ester.
- Fischer projections (Section 25.2): A means of depicting the absolute configuration of a chiral molecule on a flat page. A Fischer projection uses a cross to represent the chirality center. The horizontal arms of the cross represent bonds coming out of the plane of the page, and the vertical arms of the cross represent bonds going back into the plane of the page.
- Fmoc derivative (Section 26.7): A fluorenylmethyloxycarbonyl N-protected amino acid.
- Formal charges (Section 2.3): The difference in the number of electrons owned by an atom in a molecule and by the same atom in its elemental state.
- Formyl (Section 19.1): A −CHO group.
- Frequency, ν (Section 12.5): The number of electromagnetic wave cycles that travel past a fixed point in a given unit of time. Frequencies are expressed in units of cycles per second, or hertz.
- Friedel–Crafts reaction (Section 16.3): An electrophilic aromatic substitution reaction to alkylate or acylate an aromatic ring.
- Frontier orbitals (Section 30.1): The highest occupied (HOMO) and lowest unoccupied (LUMO) molecular orbitals.
- FT-NMR (Section 13.10): Fourier-transform NMR; a rapid technique for recording NMR spectra in which all magnetic nuclei absorb at the same time.
- Functional (Section 3.1): An atom or group of atoms that is part of a larger molecule and has a characteristic chemical reactivity.
- Functional RNAs (Section 28.4): An alternative name for small RNAs.
- Furanose (Section 25.5): The five-membered-ring form of a simple sugar.
- Gabriel amine synthesis (Section 24.6): A method for preparing an amine by SN2 reaction of an alkyl halide with potassium phthalimide, followed by hydrolysis.
- Gauche conformation (Section 3.7): The conformation of butane in which the two methyl groups lie 60° apart as viewed in a Newman projection. This conformation has 3.8 kJ/mol steric strain.
- Geminal (Section 19.5): Referring to two groups attached to the same carbon atom. For example, the hydrate formed by nucleophilic addition of water to an aldehyde or ketone is a geminal diol.
- Gibbs free-energy change (ΔG) (Section 6.7): The free-energy change that occurs during a reaction, given by the equation Δ𝐺 = Δ𝐻 − 𝑇Δ𝑆. A reaction with a negative free-energy change is spontaneous, and a reaction with a positive free-energy change is nonspontaneous.
- Gilman reagent (LiR2Cu) (Section 10.7): A diorganocopper reagent.
- Glass transition temperature, Tg (Section 31.7): The temperature at which a hard, amorphous polymer becomes soft and flexible.
- Globular proteins (Section 26.9): A type of protein that is coiled into a compact, nearly spherical shape. Globular proteins, which are generally water-soluble and mobile within the cell, are the structural class to which enzymes belong.
- Gluconeogenesis (Section 29.8): The anabolic pathway by which organisms make glucose from simple three-carbon precursors.
- Glycal (Section 25.9): An unsaturated sugar with a C1–C2 double bond.
- Glycal assembly method (Section 25.9): A method for linking monosaccharides together to synthesize polysaccharides.
- Glycerophospholipids (Section 27.3): Lipids that contain a glycerol backbone linked to two fatty acids and a phosphoric acid.
- Glycoconjugate (Section 25.6): A molecule in which a carbohydrate is linked through its anomeric center to another biological molecule such as a lipid or protein.
- Glycol (Section 8.7): A diol, such as ethylene glycol, HOCH2CH2OH.
- Glycolipid (Section 25.6): A biological molecule in which a carbohydrate is linked through a glycoside bond to a lipid.
- Glycolysis (Section 29.5): A series of ten enzyme-catalyzed reactions that break down glucose into 2 equivalents of pyruvate, CH3COCO2−.
- Glycoprotein (Section 25.6): A biological molecule in which a carbohydrate is linked through a glycoside bond to a protein.
- Glycoside (Section 25.6): A cyclic acetal formed by reaction of a sugar with another alcohol.
- Graft copolymers (Section 31.3): Copolymers in which homopolymer branches of one monomer unit are “grafted” onto a homopolymer chain of another monomer unit.
- Green chemistry (Chapter 11 Chemistry Matters, Chapter 24 Chemistry Matters): The design and implementation of chemical products and processes that reduce waste and minimize or eliminate the generation of hazardous substances.
- Grignard reagent (RMgX) (Section 10.6): An organomagnesium halide.
- Ground-state electron configuration (Section 1.3): The most stable, lowest- energy electron configuration of a molecule or atom.
- Haloform reaction (Section 22.6): The reaction of a methyl ketone with halogen and base to yield a haloform (CHX3) and a carboxylic acid.
- Halogenation (Section 8.2, Section 16.1): The reaction of halogen with an alkene to yield a 1,2-dihalide addition product or with an aromatic compound to yield a substitution product.
- Halohydrin (Section 8.3): A 1,2-haloalcohol, such as that obtained on addition of HOBr to an alkene.
- Halonium ion (Section 8.2): A species containing a positively charged, divalent halogen. Three-membered-ring bromonium ions are intermediates in the electrophilic addition of Br2 to alkenes.
- Hammond postulate (Section 7.10): A postulate stating that we can get a picture of what a given transition state looks like by looking at the structure of the nearest stable species. Exergonic reactions have transition states that resemble reactant; endergonic reactions have transition states that resemble product.
- Heat of combustion (Section 4.3): The amount of heat released when a compound burns completely in oxygen.
- Heat of hydrogenation (Section 7.6): The amount of heat released when a carbon– carbon double bond is hydrogenated.
- Heat of reaction (Section 6.7): An alternative name for the enthalpy change in a reaction, ΔH.
- Hell–Volhard–Zelinskii (HVZ) reaction (Section 22.4): The reaction of a carboxylic acid with Br2 and phosphorus to give an α-bromo carboxylic acid.
- Hemiacetal (Section 19.10): A functional group having one −OR and one −OH group bonded to the same carbon.
- Henderson–Hasselbalch equation (Section 20.3, Section 24.5, Section 26.2): An equation for determining the extent of dissociation of a weak acid at various pH values.
- Hertz, Hz (Section 12.5): A unit of measure of electromagnetic frequency, the number of waves that pass by a fixed point per second.
- Heterocycle (Section 15.5, Section 24.9): A cyclic molecule whose ring contains more than one kind of atom. For example, pyridine is a heterocycle that contains five carbon atoms and one nitrogen atom in its ring.
- Heterolytic bond breakage (Section 6.2): The kind of bond-breaking that occurs in polar reactions when one fragment leaves with both of the bonding electrons: A : B → A+ + B:−.
- Hofmann elimination reaction (Section 24.7): The elimination reaction of an amine to yield an alkene by reaction with iodomethane followed by heating with Ag2O.
- Hofmann rearrangement (Section 24.6): The conversion of an amide into an amine by reaction with Br2 and base.
- Highest occupied molecular orbital (HOMO) (Section 14.7, Section 30.1): The symmetries of the HOMO and LUMO are important in pericyclic reactions.
- Homolytic bond breakage (Section 6.2): The kind of bond-breaking that occurs in radical reactions when each fragment leaves with one bonding electron: A : B → A+ + B:−.
- Homopolymers (Section 31.3): A polymer made up of identical repeating units.
- Homotopic (Section 13.7): Hydrogens in a molecule that give the identical structure on replacement by X and thus show identical NMR absorptions.
- Hormones (Section 27.6): Chemical messengers that are secreted by an endocrine gland and carried through the bloodstream to a target tissue.
- HPLC (Section 26.5): High-pressure liquid chromatography; a variant of column chromatography using high pressure to force solvent through very small absorbent particles.
- Hückel 4n + 2 rule (Section 15.3): A rule stating that monocyclic conjugated molecules having 4n + 2 π electrons (n = an integer) are aromatic.
- Hund’s rule (Section 1.3): If two or more empty orbitals of equal energy are available, one electron occupies each, with their spins parallel, until all are half-full.
- Hybrid orbital (Section 1.6): An orbital derived from a combination of atomic orbitals. Hybrid orbitals, such as the sp3, sp2, and sp hybrids of carbon, are strongly directed and form stronger bonds than atomic orbitals do.
- Hydration (Section 8.4): Addition of water to a molecule, such as occurs when alkenes are treated with aqueous sulfuric acid to give alcohols.
- Hydride shift (Section 7.11): The shift of a hydrogen atom and its electron pair to a nearby cationic center.
- Hydroboration (Section 8.5): Addition of borane (BH3) or an alkylborane to an alkene. The resultant trialkylborane products can be oxidized to yield alcohols.
- Hydrocarbons (Section 3.2): A class of compounds that contain only carbon and hydrogen.
- Hydrogen bond (Section 2.12): A weak attraction between a hydrogen atom bonded to an electronegative atom and an electron lone pair on another electronegative atom.
- Hydrogenated (Section 6.11): Addition of hydrogen to a double or triple bond to yield a saturated product.
- Hydrogenolysis (Section 26.7): Cleavage of a bond by reaction with hydrogen. Benzylic ethers and esters, for instance, are cleaved by hydrogenolysis.
- Hydrophilic (Section 2.12): Water-loving; attracted to water.
- Hydrophobic (Section 2.12): Water-fearing; repelled by water.
- Hydroquinones (Section 17.10): 1,4-dihydroxybenzene.
- Hydroxylation (Section 8.7): Addition of two −OH groups to a double bond.
- Hyperconjugation (Section 7.6, Section 7.9): An electronic interaction that results from overlap of a vacant p orbital on one atom with a neighboring C−H σ bond. Hyperconjugation is important in stabilizing carbocations and substituted alkenes.
- Imide (Section 24.6): A compound with the −CONHCO− functional group.
- Imines (Section 19.8): A class of compounds with the R%C═NR functional group.
- Inductive effect (Section 2.1, Section 7.9, Section 16.4): The electron-attracting or electron-withdrawing effect transmitted through σ bonds. Electronegative elements have an electron-withdrawing inductive effect.
- Infrared (IR) spectroscopy (Section 12.6): A kind of optical spectroscopy that uses infrared energy. IR spectroscopy is particularly useful in organic chemistry for determining the kinds of functional groups present in molecules.
- Initiator (Section 8.10, Section 31.1): A substance that is used to initiate a radical chain reaction or polymerization. For example, radical chlorination of alkanes is initiated when light energy breaks the weak Cl−Cl bond to form Cl· radicals.
- Integrating (Section 13.5): A technique for measuring the area under an NMR peak to determine the relative number of each kind of proton in a molecule.
- Intermediate (Section 6.10): A species that is formed during the course of a multistep reaction but is not the final product. Intermediates are more stable than transition states but may or may not be stable enough to isolate.
- Intramolecular, intermolecular (Section 23.6): A reaction that occurs within the same molecule is intramolecular; a reaction that occurs between two molecules is intermolecular.
- Intron (Section 28.4): A section of DNA that does not contain genetic information.
- Ion pairs (Section 11.4): A loose association between two ions in solution. Ion pairs are implicated as intermediates in SN1 reactions to account for the partial retention of stereochemistry that is often observed.
- Ionic bond (Section 1.4): The electrostatic attraction between ions of unlike charge.
- Isoelectric point (pI) (Section 26.2): The pH at which the number of positive charges and the number of negative charges on a protein or an amino acid are equal.
- Isomers (Section 3.2, Section 5.9): Compounds that have the same molecular formula but different structures.
- Isoprene rule (Chapter 8 Chemistry Matters): An observation to the effect that terpenoids appear to be made up of isoprene (2-methyl-1,3-butadiene) units connected head-to-tail.
- Isotactic (Section 31.2): A chain-growth polymer in which the stereochemistry of the substituents is oriented regularly along the backbone.
- Isotopes (Section 1.1): Atoms of the same element that have different mass numbers.
- IUPAC system of nomenclature (Section 3.4): Rules for naming compounds, devised by the International Union of Pure and Applied Chemistry.
- Kekulé structure (Section 1.4): An alternative name for a line-bond structure, which represents a molecule by showing covalent bonds as lines between atoms.
- Ketals (Section 19.10): An alternative name for acetals derived from a ketone rather than an aldehyde and consisting of two −OR groups bonded to the same carbon, R2C(OR′)2. Ketals are often used as protecting groups for ketones.
- Keto–enol tautomerism (Section 9.4, Section 22.1): The equilibration between a carbonyl form and vinylic alcohol form of a molecule.
- Ketones (R2CO) (Section 3.1, Chapter 19 Introduction): A class of compounds with two organic substituents bonded to a carbonyl group, R%C═O.
- Ketoses (Section 25.1): Carbohydrates with a ketone functional group.
- Kiliani–Fischer synthesis (Section 25.6): A method for lengthening the chain of an aldose sugar.
- Kinetic control (Section 14.3): A reaction that follows the lowest activation energy pathway is said to be kinetically controlled. The product is the most rapidly formed but is not necessarily the most stable.
- Kinetics (Section 11.2): Referring to reaction rates. Kinetic measurements are useful for helping to determine reaction mechanisms.
- Koenigs–Knorr reaction (Section 25.6): A method for the synthesis of glycosides by reaction of an alcohol with a pyranosyl bromide.
- Krebs cycle (Section 29.7): An alternative name for the citric acid cycle, by which acetyl CoA is degraded to CO2.
- L Sugar (Section 25.3): A sugar whose hydroxyl group at the chirality center farthest from the carbonyl group points to the left when drawn in Fischer projection.
- Lactams (Section 21.7): Cyclic amides.
- Lactones (Section 21.6): Cyclic esters.
- Lagging strand (Section 28.3): The complement of the original 3′ → 5′ DNA strand that is synthesized discontinuously in small pieces that are subsequently linked by DNA ligases.
- LD50 (Chapter 1 Chemistry Matters): The amount of a substance per kilogram body weight that is lethal to 50% of test animals.
- LDA (Section 22.5): Lithium diisopropylamide, LiN(i-C3H7)2, a strong base commonly used to convert carbonyl compounds into their enolate ions.
- Leading strand (Section 28.3): The complement of the original 5′ → 3′ DNA strand that is synthesized continuously in a single piece.
- Leaving group (Section 11.3): The group that is replaced in a substitution reaction.
- Levorotatory (Section 5.3): An optically active substance that rotates the plane of polarization of plane-polarized light in a left-handed (counterclockwise) direction.
- Lewis acid (Section 2.11): A substance with a vacant low-energy orbital that can accept an electron pair from a base. All electrophiles are Lewis acids.
- Lewis base (Section 2.11): A substance that donates an electron lone pair to an acid. All nucleophiles are Lewis bases.
- Lewis structures (Section 1.4): Representations of molecules showing valence electrons as dots.
- Lindlar catalyst (Section 9.5): A hydrogenation catalyst used to convert alkynes to cis alkenes.
- Line-bond structure (Section 1.4): An alternative name for a Kekulé structure, which represents a molecule by showing covalent bonds as lines between atoms.
- 1→4 Link (Section 25.8): A glycoside link between the C1 −OH group of one sugar and the C4 −OH group of another sugar.
- Lipids (Chapter 27 Introduction): Naturally occurring substances isolated from cells and tissues by extraction with a nonpolar solvent. Lipids belong to many different structural classes, including fats, terpenoids, prostaglandins, and steroids.
- Lipid bilayer (Section 27.3): The ordered lipid structure that forms a cell membrane.
- Lipoprotein (Chapter 27 Chemistry Matters): A complex molecule with both lipid and protein parts that transports lipids through the body.
- Locant (Section 3.4): A number in a chemical name that locates the positions of the functional groups and substituents in the molecule.
- Lone-pair electrons (Section 1.4): Nonbonding valence-shell electron pairs. Lone- pair electrons are used by nucleophiles in their reactions with electrophiles.
- Lowest unoccupied molecular orbital (LUMO) (Section 14.7, Section 30.1): The symmetries of the LUMO and the HOMO are important in determining the stereochemistry of pericyclic reactions.
- Magnetic resonance imaging, MRI (Chapter 13 Chemistry Matters): A medical diagnostic technique based on nuclear magnetic resonance.
- Magnetogyric ratio (Section 13.1): A ratio of the isotope’s magnetic moment to its angular momentum.
- MALDI (Section 12.4): Matrix-assisted laser desorption ionization; a soft ionization method used for mass spectrometry of biological samples of very high molecular weight.
- Malonic ester synthesis (Section 22.7): The synthesis of a carboxylic acid by alkylation of an alkyl halide with diethyl malonate, followed by hydrolysis and decarboxylation.
- Markovnikov’s rule (Section 7.8): A guide for determining the regiochemistry (orientation) of electrophilic addition reactions. In the addition of HX to an alkene, the hydrogen atom bonds to the alkene carbon that has fewer alkyl substituents.
- Mass number (A) (Section 1.1): The total of protons plus neutrons in an atom.
- Mass spectrometry (MS) (Section 12.1): A technique for measuring the mass, and therefore the molecular weight (MW), of ions.
- McLafferty rearrangement (Section 12.3, Section 19.4): A mass-spectral fragmentation pathway for carbonyl compounds.
- Mechanism (Section 6.2): A complete description of how a reaction occurs. A mechanism accounts for all starting materials and all products and describes the details of each individual step in the overall reaction process.
- Meisenheimer complex (Section 16.6): An intermediate formed by addition of a nucleophile to a halo-substituted aromatic ring.
- Melt transition temperature, Tm (Section 31.7): The temperature at which crystalline regions of a polymer melt to give an amorphous material.
- Mercapto group (Section 18.7): An alternative name for the thiol group, −SH.
- Meso compounds (Section 5.7): Compounds that contain chirality centers but are nevertheless achiral because they contain a symmetry plane.
- Messenger RNA (mRNA) (Section 28.4): A kind of RNA formed by transcription of DNA and used to carry genetic messages from DNA to ribosomes.
- Meta (m) (Section 15.1): A naming prefix used for 1,3-disubstituted benzenes.
- Metabolism (Section 29.1): A collective name for the many reactions that go on in the cells of living organisms.
- Metallacycle (Section 31.5): A cyclic compound that contains a metal atom in its ring.
- Methylene group (Section 7.3): A −CH2− or ═CH% group.
- Micelles (Section 27.2): Spherical clusters of soaplike molecules that aggregate in aqueous solution. The ionic heads of the molecules lie on the outside, where they are solvated by water, and the organic tails bunch together on the inside of the micelle.
- Michael reaction (Section 23.10): The conjugate addition reaction of an enolate ion to an unsaturated carbonyl compound.
- Molar absorptivity (ɛ) (Section 14.7): A quantitative measure of the amount of UV light absorbed by a sample.
- Molecular ion (Section 12.1): The cation produced in a mass spectrometer by loss of an electron from the parent molecule. The mass of the molecular ion corresponds to the molecular weight of the sample.
- Molecular mechanics (Chapter 4 Chemistry Matters): A computer-based method for calculating the minimum-energy conformation of a molecule.
- Molecular orbital (MO) theory (Section 1.11, Section 14.1): A description of covalent bond formation as resulting from a mathematical combination of atomic orbitals (wave functions) to form molecular orbitals.
- Molecule (Section 1.4): A neutral collection of atoms held together by covalent bonds.
- Molozonide (Section 8.8): The initial addition product of ozone with an alkene.
- Monomers (Section 8.10, Section 21.9; Chapter 31 Introduction): The simple starting units from which polymers are made.
- Monosaccharides (Section 25.1): Simple sugars.
- Monoterpenoids (Chapter 8 Chemistry Matters, Section 27.5): Ten-carbon lipids.
- Multiplet (Section 13.6): A pattern of peaks in an NMR spectrum that arises by spin–spin splitting of a single absorption because of coupling between neighboring magnetic nuclei.
- Mutarotation (Section 25.5): The change in optical rotation observed when a pure anomer of a sugar is dissolved in water. Mutarotation is caused by the reversible opening and closing of the acetal linkage, which yields an equilibrium mixture of anomers.
- n + 1 rule (Section 13.6): A hydrogen with n other hydrogens on neighboring carbons shows n + 1 peaks in its 1H NMR spectrum.
- N-terminal amino acid (Section 26.4): The amino acid with a free −NH2 group at the end of a protein chain.
- Natural gas (Chapter 3 Chemistry Matters): A naturally occurring hydrocarbon mixture consisting chiefly of methane, along with smaller amounts of ethane, propane, and butane.
- Natural product (Chapter 7 Chemistry Matters): A catchall term generally taken to mean a secondary metabolite found in bacteria, plants, and other living organisms.
- New molecular entity, NME (Chapter 6 Chemistry Matters): A new biologically active chemical substance approved for sale as a drug by the U.S. Food and Drug Administration.
- Newman projection (Section 3.6): A means of indicating stereochemical relationships between substituent groups on neighboring carbons. The carbon– carbon bond is viewed end-on, and the carbons are indicated by a circle. Bonds radiating from the center of the circle are attached to the front carbon, and bonds radiating from the edge of the circle are attached to the rear carbon.
- Nitration (Section 16.2): The substitution of a nitro group onto an aromatic ring.
- Nitriles (Section 3.1, Section 20.1): A class of compounds containing the C≡N functional group.
- Nitrogen rule (Section 24.10): A compound with an odd number of nitrogen atoms has an odd-numbered molecular weight.
- Node (Section 1.2): A surface of zero electron density within an orbital. For example, a p orbital has a nodal plane passing through the center of the nucleus, perpendicular to the axis of the orbital.
- Nonbonding electrons (Section 1.4): Valence electrons that are not used in forming covalent bonds.
- Noncoding strand (Section 28.4): An alternative name for the antisense strand of DNA.
- Noncovalent interactions (Section 2.12): One of a variety of nonbonding interactions between molecules, such as dipole–dipole forces, dispersion forces, and hydrogen bonds.
- Nonessential amino acid (Section 26.1): One of the eleven amino acids that are biosynthesized by humans.
- Normal alkanes (Section 3.2): Straight-chain alkanes, as opposed to branched alkanes. Normal alkanes are denoted by the suffix n, as in n-C4H10 (n-butane).
- NSAID (Chapter 15 Chemistry Matters): A nonsteroidal anti-inflammatory drug, such as aspirin or ibuprofen.
- Nuclear magnetic resonance (NMR) spectroscopy (Chapter 13 Introduction): A spectroscopic technique that provides information about the carbon–hydrogen framework of a molecule. NMR works by detecting the energy absorptions accompanying the transitions between nuclear spin states that occur when a molecule is placed in a strong magnetic field and irradiated with radiofrequency waves.
- Nucleic acid (Section 28.1): Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA); biological polymers made of nucleotides joined together to form long chains.
- Nucleophile (Section 6.3, Section 6.4): An electron-rich species that donates an electron pair to an electrophile in a polar bond-forming reaction. Nucleophiles are also Lewis bases.
- Nucleophilic acyl substitution reaction (Section 21.2): A reaction in which a nucleophile attacks a carbonyl compound and substitutes for a leaving group bonded to the carbonyl carbon.
- Nucleophilic addition reaction (Section 19.4): A reaction in which a nucleophile adds to the electrophilic carbonyl group of a ketone or aldehyde to give an alcohol.
- Nucleophilic aromatic substitution reactions (Section 16.6): The substitution reactions of an aryl halide by a nucleophile.
- Nucleophilic substitution reactions (Section 11.1): Reactions in which one nucleophile replaces another attached to a saturated carbon atom.
- Nucleophilicity (Section 11.3): The ability of a substance to act as a nucleophile in an SN2 reaction.
- Nucleoside (Section 28.1): A nucleic acid constituent consisting of a sugar residue bonded to a heterocyclic purine or pyrimidine base.
- Nucleotides (Section 28.1): Nucleic acid constituents consisting of a sugar residue bonded both to a heterocyclic purine or pyrimidine base and to a phosphoric acid. Nucleotides are the monomer units from which DNA and RNA are constructed.
- Nylons (Section 21.9): Synthetic polyamide step-growth polymers.
- Okazaki fragments (Section 28.3): Short segments of a DNA lagging strand that is biosynthesized discontinuously and then linked by DNA ligases.
- Olefin (Chapter 7 Introduction): An alternative name for an alkene.
- Olefin metathesis polymerization (Section 31.5): A method of polymer synthesis based on using an olefin metathesis reaction.
- Olefin metathesis reaction (Section 31.5): A reaction in which two olefins (alkenes) exchange substituents on their double bonds.
- Oligonucleotides (Section 28.7): Short segments of DNA.
- Optical isomers (Section 5.4): An alternative name for enantiomers. Optical isomers are isomers that have a mirror-image relationship.
- Optically active (Section 5.3): A property of some organic molecules wherein the plane of polarization is rotated through an angle when a beam of plane-polarized light is passed through a solution of the molecules.
- Orbital (Section 1.2): A wave function, which describes the volume of space around a nucleus in which an electron is most likely to be found.
- Organic chemistry (Chapter 1 Introduction): The study of carbon compounds.
- Organohalides (Chapter 10 Introduction): Compounds that contain one or more halogen atoms bonded to carbon.
- Organometallic compound (Section 10.6): A compound that contains a carbon– metal bond. Grignard reagents, RMgX, are examples.
- Organophosphate (Section 1.10): A compound that contains a phosphorus atom bonded to four oxygens, with one of the oxygens also bonded to carbon.
- Ortho (o) (Section 15.1): A naming prefix used for 1,2-disubstituted benzenes.
- Oxidation (Section 8.7, Section 10.8): A reaction that causes a decrease in electron ownership by carbon, either by bond formation between carbon and a more electronegative atom (usually oxygen, nitrogen, or a halogen) or by bond-breaking between carbon and a less electronegative atom (usually hydrogen).
- Oximes (Section 19.8): Compounds with the R%C═NOH functional group.
- Oxirane (Section 8.7): An alternative name for an epoxide.
- Oxymercuration (Section 8.4): A method for double-bond hydration by reaction of an alkene with aqueous mercuric acetate followed by treatment with NaBH4.
- Ozonide (Section 8.8): The product initially formed by addition of ozone to a carbon–carbon double bond. Ozonides are usually treated with a reducing agent, such as zinc in acetic acid, to produce carbonyl compounds.
- Para (p) (Section 15.1): A naming prefix used for 1,4-disubstituted benzenes.
- Paraffins (Section 3.5): A common name for alkanes.
- Parent peak (Section 12.1): The peak in a mass spectrum corresponding to the molecular ion. The mass of the parent peak therefore represents the molecular weight of the compound.
- Pauli exclusion principle (Section 1.3): No more than two electrons can occupy the same orbital, and those two must have spins of opposite sign.
- Peptide bond (Section 26.4): An amide bond in a peptide chain.
- Peptides (Chapter 26 Introduction): A type of short amino acid polymer in which the individual amino acid residues are linked by amide bonds.
- Pericyclic reaction (Chapter 30 Introduction): A reaction that occurs in a single step by a reorganization of bonding electrons in a cyclic transition state.
- Periplanar (Section 11.8): A conformation in which bonds to neighboring atoms have a parallel arrangement. In an eclipsed conformation, the neighboring bonds are syn periplanar; in a staggered conformation, the bonds are anti periplanar.
- Peroxides (Section 18.1): Molecules containing an oxygen–oxygen bond functional group, ROOR′ or ROOH.
- Peroxyacid (Section 8.7): A compound with the −CO3H functional group. Peroxyacids react with alkenes to give epoxides.
- Phenols (Chapter 17 Introduction): A class of compounds with an −OH group directly bonded to an aromatic ring, ArOH.
- Phenoxide ion, ArO− (Section 17.2): The anion of a phenol.
- Phenyl (Section 15.1): The name for the −C6H5 unit when the benzene ring is considered as a substituent. A phenyl group is abbreviated as −Ph.
- Phosphine (Section 5.10): A trivalent phosphorus compound, R3P.
- Phosphite (Section 28.7): A compound with the structure P(OR)3.
- Phospholipids (Section 27.3): Lipids that contain a phosphate residue. For example, glycerophospholipids contain a glycerol backbone linked to two fatty acids and a phosphoric acid.
- Phosphoramidite (Section 28.7): A compound with the structure R2NP(OR)2.
- Phosphoric acid anhydride (Section 29.1): A substance that contains PO2PO link, analogous to the CO2CO link in carboxylic acid anhydrides.
- Photochemical reactions (Section 30.3): A reaction carried out by irradiating the reactants with light.
- Physiological pH (Section 20.3): The pH of 7.3 that exists inside cells.
- Pi (π) bond (Section 1.8): The covalent bond formed by sideways overlap of atomic orbitals. For example, carbon–carbon double bonds contain a π bond formed by sideways overlap of two p orbitals.
- PITC (Section 26.6): Phenylisothiocyanate; used in the Edman degradation.
- pKa (Section 2.8): The negative common logarithm of the Ka; used to express acid strength.
- Plane of symmetry (Section 5.2): A plane that bisects a molecule such that one half of the molecule is the mirror image of the other half. Molecules containing a plane of symmetry are achiral.
- Plane-polarized light (Section 5.3): Light that has its electromagnetic waves oscillating in a single plane rather than in random planes. The plane of polarization is rotated when the light is passed through a solution of a chiral substance.
- Plasticizers (Section 31.7): Small organic molecules added to polymers to act as a lubricant between polymer chains.
- Polar aprotic solvents (Section 11.3): Polar solvents that can’t function as hydrogen ion donors. Polar aprotic solvents such as dimethyl sulfoxide (DMSO) and dimethylformamide (DMF) are particularly useful in SN2 reactions because of their ability to solvate cations.
- Polar covalent bond (Section 2.1): A covalent bond in which the electron distribution between atoms is unsymmetrical.
- Polar reactions (Section 6.2, Section 6.3): Reactions in which bonds are made when a nucleophile donates two electrons to an electrophile and in which bonds are broken when one fragment leaves with both electrons from the bond.
- Polarity (Section 2.1): The unsymmetrical distribution of electrons in a molecule that results when one atom attracts electrons more strongly than another.
- Polarizability (Section 6.3): The measure of the change in a molecule’s electron distribution in response to changing electrostatic interactions with solvents or ionic reagents.
- Polycarbonates (Section 31.4): Polyesters in which the carbonyl groups are linked to two −OR groups, [O═C(OR)%].
- Polycyclic (Section 4.9): Containing more than one ring.
- Polycyclic aromatic compound (Section 15.6): A compound with two or more benzene-like aromatic rings fused together.
- Polymer (Section 8.10, Section 21.9; Chapter 31 Introduction): A large molecule made up of repeating smaller units. For example, polyethylene is a synthetic polymer made from repeating ethylene units, and DNA is a biopolymer made of repeating deoxyribonucleotide units.
- Polymerase chain reaction (PCR) (Section 28.8): A method for amplifying small amounts of DNA to produce larger amounts.
- Polysaccharides (Section 25.9): A type of carbohydrate that is made of many simple sugars linked together by glycoside (acetal) bonds.
- Polyunsaturated fatty acids (Section 27.1): Fatty acids that contain more than one double bond.
- Polyurethane (Section 31.4): A step-growth polymer prepared by reaction between a diol and a diisocyanate.
- Posttranslational modification (Section 28.6): A chemical modification of a protein that occurs after translation from DNA.
- Primary, secondary, tertiary, and quaternary (Section 3.3): Terms used to describe the substitution pattern at a specific site. A primary site has one organic substituent attached to it, a secondary site has two organic substituents, a tertiary site has three, and a quaternary site has four.
- Primary structure (Section 26.9): The amino acid sequence in a protein.
- pro-R (Section 5.11): One of two identical atoms or groups of atoms in a compound whose replacement leads to an R chirality center.
- pro-S (Section 5.11): One of two identical atoms or groups of atoms in a compound whose replacement leads to an S chirality center.
- Prochiral (Section 5.11): A molecule that can be converted from achiral to chiral in a single chemical step.
- Prochirality center (Section 5.11): An atom in a compound that can be converted into a chirality center by changing one of its attached substituents.
- Promoter sequence (Section 28.4): A short sequence on DNA located upstream of the transcription start site and recognized by RNA polymerase.
- Propagation step (Section 8.10): A step in a radical chain reaction that carries on the chain. The propagation steps must yield both product and a reactive intermediate.
- Prostaglandins (Section 27.4): Lipids derived from arachidonic acid. Prostaglandins are present in nearly all body tissues and fluids, where they serve many important hormonal functions.
- Protecting group (Section 17.8, Section 19.10, Section 26.7): A group that is introduced to protect a sensitive functional group toward reaction elsewhere in the molecule. After serving its protective function, the group is removed.
- Proteins (Chapter 26 Introduction): Large peptides containing 50 or more amino acid residues. Proteins serve both as structural materials and as enzymes that control an organism’s chemistry.
- Protein Data Bank (Chapter 26 Chemistry Matters): A worldwide online repository of X-ray and NMR structural data for biological macromolecules. To access the Protein Data Bank, go to https://www.rcsb.org.
- Protic solvents (Section 11.3): Solvents such as water or alcohol that can act as a proton donor.
- Pyramidal inversion (Section 24.2): The rapid stereochemical inversion of a trivalent nitrogen compound.
- Pyranose (Section 25.5): The six-membered, cyclic hemiacetal form of a simple sugar.
- Quadrupole mass analyzer (Section 12.1): A type of mass spectrometer that uses four cylindrical rods to create an oscillating electrostatic field. Ion trajectories are determined by their m/z ratios. At a given field, only one m/z value will make it through the quadrupole region—the others will crash into the quadrupole rods or the walls of the instrument and never reach the detector.
- Quartet (Section 13.6): A set of four peaks in an NMR spectrum, caused by spin– spin splitting of a signal by three adjacent nuclear spins.
- Quaternary ammonium salt (Section 24.1): An ionic compound containing a positively charged nitrogen atom with four attached groups, R4N+ X−.
- Quaternary structure (Section 26.9): The highest level of protein structure, involving an ordered aggregation of individual proteins into a larger cluster.
- Quinone (Section 17.10): A 2,5-cyclohexadiene-1,4-dione.
- R configuration (Section 5.5): The configuration at a chirality center as specified using the Cahn–Ingold–Prelog sequence rules.
- R (Section 3.3): A generalized abbreviation for an organic partial structure.
- Racemate (Section 5.8): A mixture consisting of equal parts (+) and (−) enantiomers of a chiral substance; also called a racemic mixture.
- Radical (Section 2.6, Section 6.2): A species that has an odd number of electrons, such as the chlorine radical, Cl·.
- Radical reactions (Section 6.2, Section 6.3): Reactions in which bonds are made by donation of one electron from each of two reactants and in which bonds are broken when each fragment leaves with one electron.
- Rate constant (Section 11.2): The constant k in a rate equation.
- Rate equation (Section 11.2): An equation that expresses the dependence of a reaction’s rate on the concentration of reactants.
- Rate-limiting step (Section 11.4): The slowest step in a multistep reaction sequence; also called the rate-determining step. The rate-limiting step acts as a kind of bottleneck in multistep reactions.
- Re face (Section 5.11): One of two faces of a planar, sp2-hybridized atom.
- Rearrangement reactions (Section 6.1): What occurs when a single reactant undergoes a reorganization of bonds and atoms to yield an isomeric product.
- Reducing sugars (Section 25.6): Sugars that reduce silver ion in the Tollens test or cupric ion in the Fehling or Benedict tests.
- Reduction (Section 8.6, Section 10.8): A reaction that causes an increase of electron ownership by carbon, either by bond-breaking between carbon and a more electronegative atom or by bond formation between carbon and a less electronegative atom.
- Reductive amination (Section 24.6, Section 26.3): A method for preparing an amine by reaction of an aldehyde or ketone with ammonia and a reducing agent.
- Refining (Chapter 3 Chemistry Matters): The process by which petroleum is converted into gasoline and other useful products.
- Regiochemistry (Section 7.8): A term describing the orientation of a reaction that occurs on an unsymmetrical substrate.
- Regiospecific (Section 7.8): A term describing a reaction that occurs with a specific regiochemistry to give a single product rather than a mixture of products.
- Replication (Section 28.3): The process by which double-stranded DNA uncoils and is replicated to produce two new copies.
- Replication forks (Section 28.3): The point of unraveling in a DNA chain where replication occurs.
- Residues (Section 26.4): Amino acids in a protein chain.
- Resolution (Section 5.8): The process by which a racemate is separated into its two pure enantiomers.
- Resonance effect (Section 16.4): The donation or withdrawal of electrons through orbital overlap with neighboring π bonds. For example, an oxygen or nitrogen substituent donates electrons to an aromatic ring by overlap of the O or N orbital with the aromatic ring p orbitals.
- Resonance forms (Section 2.4): Individual structural forms of a resonance hybrid.
- Resonance hybrid (Section 2.4): A molecule, such as benzene, that can’t be represented adequately by a single Kekulé structure but must instead be considered as an average of two or more resonance forms. The resonance forms themselves differ only in the positions of their electrons, not their nuclei.
- Restriction endonucleases (Section 28.6): Enzymes that are able to cleave a DNA molecule at points in the chain where a specific base sequence occurs.
- Retrosynthetic (Section 9.9, Section 16.10): Planning an organic synthesis by working backward from the final product to the starting material.
- Ribonucleic acid (RNA) (Chapter 28 Introduction): The biopolymer found in cells that serves to transcribe the genetic information found in DNA and uses that information to direct the synthesis of proteins.
- Ribosomal RNA (rRNA) (Section 28.4): A kind of RNA used in the physical makeup of ribosomes.
- Ring-current (Section 15.7): The circulation of π electrons induced in aromatic rings by an external magnetic field. This effect accounts for the downfield shift of aromatic ring protons in the 1H NMR spectrum.
- Ring-flip (Section 4.6): A molecular motion that interconverts two chair conformations of cyclohexane. The effect of a ring-flip is to convert an axial substituent into an equatorial substituent.
- Ring-opening metathesis polymerization (ROMP) (Section 31.5): A method of polymer synthesis that uses an olefin metathesis reaction of a cycloalkene.
- RNA (Section 28.1): Ribonucleic acid.
- Robinson annulation reaction (Section 23.12): A method for synthesis of cyclohexenones by sequential Michael reaction and intramolecular aldol reaction.
- S configuration (Section 5.5): The configuration at a chirality center as specified using the Cahn–Ingold–Prelog sequence rules.
- s-Cis conformation (Section 14.5): The conformation of a conjugated diene that is cis-like around the single bond.
- Saccharide (Section 25.1): A sugar.
- Salt bridge (Section 26.9): An ionic attraction between two oppositely charged groups in a protein chain.
- Sandmeyer reaction (Section 24.8): The nucleophilic substitution reaction of an arenediazonium salt with a cuprous halide to yield an aryl halide.
- Sanger dideoxy method (Section 28.6): A commonly used method of DNA sequencing.
- Saponification (Section 21.6, Section 27.2): An old term for the base-induced hydrolysis of an ester to yield a carboxylic acid salt.
- Saturated (Section 3.2): A molecule that has only single bonds and thus can’t undergo addition reactions. Alkanes are saturated, but alkenes are unsaturated.
- Sawhorse representations (Section 3.6): A manner of representing stereochemistry that uses a stick drawing and gives a perspective view of the conformation around a single bond.
- Schiff bases (Section 19.8, Section 29.5): An alternative name for an imine, R%C═NR′, used primarily in biochemistry.
- Second-order reaction (Section 11.2): A reaction whose rate-limiting step is bimolecular and whose kinetics are therefore dependent on the concentration of two reactants.
- Secondary metabolite (Chapter 7 Chemistry Matters): A small naturally occurring molecule that is not essential to the growth and development of the producing organism and is not classified by structure.
- Secondary structure (Section 26.9): The level of protein substructure that involves organization of chain sections into ordered arrangements such as β-pleated sheets or α helices.
- Semiconservative replication (Section 28.3): The process by which DNA molecules are made containing one strand of old DNA and one strand of new DNA.
- Sense strand (Section 28.4): The coding strand of double-helical DNA that contains the gene.
- Sequence rules (Section 5.5, Section 7.5): A series of rules for assigning relative rankings to substituent groups on a double-bond carbon atom or on a chirality center.
- Sesquiterpenoids (Section 27.5): 15-carbon lipids.
- Sharpless epoxidation (Chapter 19 Chemistry Matters): A method for enantioselective synthesis of a chiral epoxide by treatment of an allylic alcohol with tert-butyl hydroperoxide, (CH3)3C–OOH, in the presence of titanium tetraisopropoxide and diethyl tartrate.
- Shielding (Section 13.2): An effect observed in NMR that causes a nucleus to absorb toward the right (upfield) side of the chart. Shielding is caused by donation of electron density to the nucleus.
- Si face (Section 5.11): One of two faces of a planar, sp2-hybridized atom.
- Sialic acid (Section 25.7): One of a group of more than 300 carbohydrates based on acetylneuramic acid.
- Side chain (Section 26.1): The substituent attached to the α carbon of an amino acid.
- Sigma (σ) bond (Section 1.5): A covalent bond formed by head-on overlap of atomic orbitals.
- Sigmatropic reaction (Section 30.8): A pericyclic reaction that involves the migration of a group from one end of a π electron system to the other.
- Silyl ether (Section 17.8): A substance with the structure R3Si–O–R. The silyl ether acts as a protecting group for alcohols.
- Simmons–Smith reaction (Section 8.9): The reaction of an alkene with CH2I2 and Zn−Cu to yield a cyclopropane.
- Simple sugars (Section 25.1): Carbohydrates that cannot be broken down into smaller sugars by hydrolysis.
- Single bond (Section 1.8): A covalent bond formed by sharing one electron pair between atoms.
- Skeletal structures (Section 1.12): A shorthand way of writing structures in which carbon atoms are assumed to be at each intersection of two lines (bonds) and at the end of each line.
- Small RNAs (Section 28.4): A type of RNA that has a variety of functions within the cell, including silencing transcription and catalyzing chemical modifications of other RNA molecules.
- SN1 reaction (Section 11.4): A unimolecular nucleophilic substitution reaction.
- SN2 reaction (Section 11.2): A bimolecular nucleophilic substitution reaction.
- Solid-phase synthesis (Section 26.8): A technique of synthesis whereby the starting material is covalently bound to a solid polymer bead and reactions are carried out on the bound substrate. After the desired transformations have been effected, the product is cleaved from the polymer.
- Solvation (Section 11.3): The clustering of solvent molecules around a solute particle to stabilize it.
- sp hybrid orbitals (Section 1.9): Hybrid orbitals derived from the combination of an s and a p atomic orbital. The two sp orbitals that result from hybridization are oriented at an angle of 180° to each other.
- sp2 hybrid orbitals (Section 1.8): Hybrid orbitals derived by combination of an s atomic orbital with two p atomic orbitals. The three sp2 hybrid orbitals that result lie in a plane at angles of 120° to each other.
- sp3 hybrid orbitals (Section 1.6): Hybrid orbitals derived by combination of an s atomic orbital with three p atomic orbitals. The four sp3 hybrid orbitals that result are directed toward the corners of a regular tetrahedron at angles of 109° to each other.
- Specific rotation, [α]D (Section 5.3): The optical rotation of a chiral compound under standard conditions.
- Sphingomyelins (Section 27.3): Phospholipids that have sphingosine as the backbone rather than glycerol.
- Spin–spin splitting (Section 13.6): The splitting of an NMR signal into a multiplet because of an interaction between nearby magnetic nuclei whose spins are coupled. The magnitude of spin–spin splitting is given by the coupling constant, J.
- Staggered conformation (Section 3.6): The three-dimensional arrangement of atoms around a carbon–carbon single bond in which the bonds on one carbon bisect the bond angles on the second carbon as viewed end-on.
- Statin (Chapter 29 Chemistry Matters): A drug that controls cholesterol biosynthesis in the body by blocking the HMG-CoA reductase enzyme.
- Step-growth polymers (Section 21.9, Section 31.4): Polymers in which each bond is formed independently of the others. Polyesters and polyamides (nylons) are examples.
- Stereocenter (Section 5.2): An alternative name for a chirality center.
- Stereochemistry (Section 3.5; Chapters 3, 4, 5): The branch of chemistry concerned with the three-dimensional arrangement of atoms in molecules.
- Stereogenic center (Section 5.2): An alternative name for a chirality center.
- Stereoisomers (Section 4.2): Isomers that have their atoms connected in the same order but have different three-dimensional arrangements. The term stereoisomer includes both enantiomers and diastereomers.
- Stereospecific (Section 8.9, Section 14.5): A term indicating that only a single stereoisomer is produced in a given reaction rather than a mixture.
- Steric strain (Section 3.7, Section 4.3, Section 4.7): The strain imposed on a molecule when two groups are too close together and try to occupy the same space. Steric strain is responsible both for the greater stability of trans versus cis alkenes and for the greater stability of equatorially substituted versus axially substituted cyclohexanes.
- Steroids (Section 27.6): Lipids whose structure is based on a tetracyclic carbon skeleton with three 6-membered and one 5-membered ring. Steroids occur in both plants and animals and have a variety of important hormonal functions.
- Stork enamine reaction (Section 23.11): The conjugate addition of an enamine to an α,β-unsaturated carbonyl compound, followed by hydrolysis to yield a 1,5- dicarbonyl product.
- STR loci (Chapter 28 Chemistry Matters): Short tandem repeat sequences of noncoding DNA that are unique to every individual and allow DNA fingerprinting.
- Straight-chain alkanes (Section 3.2): Alkanes whose carbon atoms are connected without branching.
- Substitution reactions (Section 6.1): What occurs when two reactants exchange parts to give two new products. SN1 and SN2 reactions are examples.
- Sulfides (Section 3.1, Section 18.7): A class of compounds that has two organic substituents bonded to the same sulfur atom, RSR′.
- Sulfonation (Section 16.2): The substitution of a sulfonic acid group (−SO3H) onto an aromatic ring.
- Sulfone (Section 18.7): A compound of the general structure RSO2R′.
- Sulfonium ions (Section 18.7): A species containing a positively charged, trivalent sulfur atom, R3S+.
- Sulfoxide (Section 18.7): A compound of the general structure RSOR′.
- Suprafacial (Section 30.5): A word used to describe the geometry of pericyclic reactions. Suprafacial reactions take place on the same side of the two ends of a π electron system.
- Suzuki–Miyaura reaction (Section 10.7): The palladium-catalyzed coupling reaction of an aromatic or vinylic halide with an aromatic or vinylic boronic acid.
- Symmetry-allowed, symmetry-disallowed (Section 30.1): A symmetry-allowed reaction is a pericyclic process that has a favorable orbital symmetry for reaction through a concerted pathway. A symmetry-disallowed reaction is one that does not have favorable orbital symmetry for reaction through a concerted pathway.
- Symmetry plane (Section 5.2): A plane that bisects a molecule such that one half of the molecule is the mirror image of the other half. Molecules containing a plane of symmetry are achiral.
- Syn periplanar (Section 11.8): Describing a stereochemical relationship in which two bonds on adjacent carbons lie in the same plane and are eclipsed.
- Syn stereochemistry (Section 8.5): The opposite of anti. A syn addition reaction is one in which the two ends of the double bond react from the same side. A syn elimination is one in which the two groups leave from the same side of the molecule.
- Syndiotactic (Section 31.2): A chain-growth polymer in which the stereochemistry of the substituents alternates regularly on opposite sides of the backbone.
- Tautomers (Section 9.4, Section 22.1): Isomers that interconvert spontaneously, usually with the change in position of a hydrogen.
- Terpenoids (Chapter 8 Chemistry Matters, Section 27.5): Lipids that are formally derived by head-to-tail polymerization of isoprene units.
- Tertiary structure (Section 26.9): The level of protein structure that involves the manner in which the entire protein chain is folded into a specific three-dimensional arrangement.
- Thermodynamic control (Section 14.3): An equilibrium reaction that yields the lowest-energy, most stable product is said to be thermodynamically controlled.
- Thermoplastics (Section 31.7): Polymers that have a high Tg and are hard at room temperature but become soft and viscous when heated.
- Thermosetting resins (Section 31.7): Polymers that become highly cross-linked and solidify into a hard, insoluble mass when heated.
- Thioesters (Chapter 21 Introduction): A class of compounds with the RCOSR′ functional group.
- Thiols (Section 3.1, Chapter 18 Introduction): A class of compounds containing the −SH functional group.
- Thiolate ion (Section 18.7): The anion of a thiol, RS−.
- TMS (Section 13.3): Tetramethylsilane; used as an NMR calibration standard.
- TOF (Section 12.4): Time-of-flight mass spectrometry; a sensitive method of mass detection accurate to about 3 ppm.
- Tollens’ reagent (Section 25.6): A solution of Ag2O in aqueous ammonia; used to oxidize aldehydes to carboxylic acids.
- Torsional strain (Section 3.6, Section 4.3): The strain in a molecule caused by electron repulsion between eclipsed bonds. Torsional strain is also called eclipsing strain.
- Tosylate (Section 11.1): A p-toluenesulfonate ester; useful as a leaving group in nucleophilic substitution reactions.
- Transamination (Section 29.9): The exchange of an amino group and a keto group between reactants.
- Transcription (Section 28.4): The process by which the genetic information encoded in DNA is read and used to synthesize RNA in the nucleus of the cell. A small portion of double-stranded DNA uncoils, and complementary ribonucleotides line up in the correct sequence for RNA synthesis.
- Transfer RNA (tRNA) (Section 28.4): A kind of RNA that transports amino acids to the ribosomes, where they are joined together to make proteins.
- Transimination (Section 29.9): The exchange of an amino group and an imine group between reactants.
- Transition state (Section 6.9): An activated complex between reactants, representing the highest energy point on a reaction curve. Transition states are unstable complexes that can’t be isolated.
- Translation (Section 28.5): The process by which the genetic information transcribed from DNA onto mRNA is read by tRNA and used to direct protein synthesis.
- Tree diagram (Section 13.8): A diagram used in NMR to sort out the complicated splitting patterns that can arise from multiple couplings.
- Triacylglycerols (Section 27.1): Lipids, such as those found in animal fat and vegetable oil, that are a triester of glycerol with long-chain fatty acids.
- Tricarboxylic acid cycle (Section 29.7): An alternative name for the citric acid cycle by which acetyl CoA is degraded to CO2.
- Triple bonds (Section 1.8): A type of covalent bond formed by sharing three electron pairs between atoms.
- Triplet (Section 13.6): A symmetrical three-line splitting pattern observed in the 1H NMR spectrum when a proton has two equivalent neighbor protons.
- Turnover number (Section 26.10): The number of substrate molecules acted on by an enzyme molecule per unit time.
- Twist-boat conformation (Section 4.5): A conformation of cyclohexane that is somewhat more stable than a pure boat conformation.
- Ultraviolet (UV) spectroscopy (Section 14.7): An optical spectroscopy employing ultraviolet irradiation. UV spectroscopy provides structural information about the extent of π electron conjugation in organic molecules.
- Unimolecular reaction (Section 11.4): A reaction that occurs by spontaneous transformation of the starting material without the intervention of other reactants. For example, the dissociation of a tertiary alkyl halide in the SN1 reaction is a unimolecular process.
- Unsaturated (Section 7.2): A molecule that has one or more multiple bonds.
- Upfield (Section 13.3): The right-hand portion of the NMR chart.
- Urethane (Section 31.4): A functional group in which a carbonyl group is bonded to both an −OR and an −NR2.
- Uronic acid (Section 25.6): A monocarboxylic acid formed by oxidizing the −CH2OH end of an aldose without affecting the −CHO end.
- Valence bond theory (Section 1.5): A bonding theory that describes a covalent bond as resulting from the overlap of two atomic orbitals.
- Valence shell (Section 1.4): The outermost electron shell of an atom.
- van der Waals forces (Section 2.12): Intermolecular forces that are responsible for holding molecules together in the liquid and solid states.
- Vegetable oils (Section 27.1): Liquid triacylglycerols derived from a plant source.
- Vicinal (Section 9.2): A term used to refer to a 1,2-disubstitution pattern. For example, 1,2-dibromoethane is a vicinal dibromide.
- Vinyl group (Section 7.3): A H%C═CH– substituent.
- Vinyl monomer (Section 8.10, Section 31.1): A substituted alkene monomer used to make a chain-growth polymer.
- Vinylic (Section 9.3): A term that refers to a substituent at a double-bond carbon atom. For example, chloroethylene is a vinylic chloride, and enols are vinylic alcohols.
- Vitamin (Section 26.10): A small organic molecule that must be obtained in the diet and is required in trace amounts for proper growth and function.
- Vulcanization (Section 14.6): A technique for cross-linking and hardening a diene polymer by heating with a few percent by weight of sulfur.
- Walden inversion (Section 11.1): The inversion of configuration at a chirality center that accompanies an SN2 reaction.
- Wave equation (Section 1.2): A mathematical expression that defines the behavior of an electron in an atom.
- Wave function (Section 1.2): A solution to the wave equation for defining the behavior of an electron in an atom. The square of the wave function defines the shape of an orbital.
- Wavelength, λ (Section 12.5): The length of a wave from peak to peak. The wavelength of electromagnetic radiation is inversely proportional to frequency and inversely proportional to energy.
- Wavenumber, 𝛎/ (Section 12.6): The reciprocal of the wavelength in centimeters.
- Waxes (Section 27.1): A mixture of esters of long-chain carboxylic acids with long-chain alcohols.
- Williamson ether synthesis (Section 18.2): A method for synthesizing ethers by SN2 reaction of an alkyl halide with an alkoxide ion.
- Wittig reaction (Section 19.11): The reaction of a phosphorus ylide with a ketone or aldehyde to yield an alkene.
- Wohl degradation (Section 25.6): A method for shortening the chain of an aldose sugar by one carbon.
- Wolff–Kishner reaction (Section 19.9): The conversion of an aldehyde or ketone into an alkane by reaction with hydrazine and base.
- X-ray crystallography (Chapter 12 Chemistry Matters): A technique that uses X rays to determine the structure of molecules.
- Ylide (Section 19.11): A neutral species with adjacent + and − charges, such as the phosphoranes used in Wittig reactions.
- Z geometry (Section 7.5): A term used to describe the stereochemistry of a carbon– carbon double bond. The two groups on each carbon are ranked according to the Cahn–Ingold–Prelog sequence rules, and the two carbons are compared. If the higher ranked groups on each carbon are on the same side of the double bond, the bond has Z geometry.
- Zaitsev’s rule (Section 11.7): A rule stating that E2 elimination reactions normally yield the more highly substituted alkene as major product.
- Ziegler–Natta catalysts (Section 31.2): Catalysts of an alkylaluminum and a titanium compound used for preparing alkene polymers.
- Zwitterion (Section 26.1): A neutral dipolar molecule in which the positive and negative charges are not adjacent. For example, amino acids exist as zwitterions, $ H& CN–CHR–CO%!

