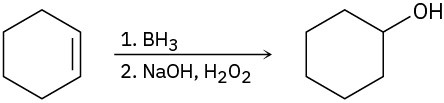
We’ve pointed out on several occasions that some of the reactions discussed in this and earlier chapters are either oxidations or reductions. As noted in Section 8.7, an organic oxidation results in a loss of electron density by carbon, caused either by bond formation between carbon and a more electronegative atom (usually O, N, or a halogen) or by bond- breaking between carbon and a less electronegative atom (usually H). Conversely, an organic reduction results in a gain of electron density by carbon, caused either by bond formation between carbon and a less electronegative atom or by bond-breaking between carbon and a more electronegative atom (Section 8.6).

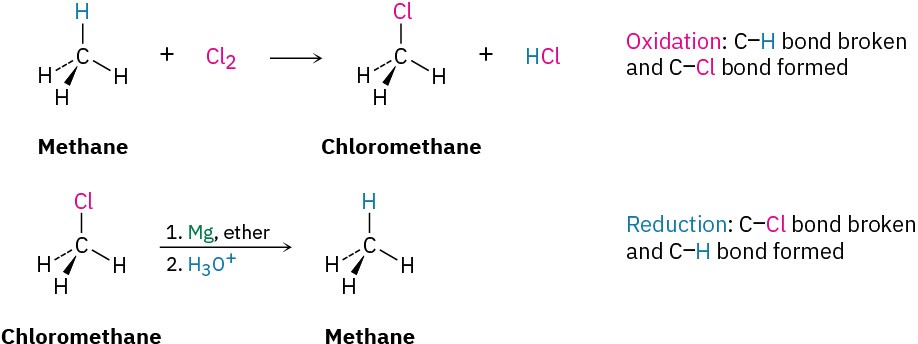
Based on these definitions, the chlorination reaction of methane to yield chloromethane is an oxidation because a C−H bond is broken and a C−Cl bond is formed. The conversion of an alkyl chloride to an alkane via a Grignard reagent followed by protonation is a reduction, however, because a C−Cl bond is broken and a C−H bond is formed.
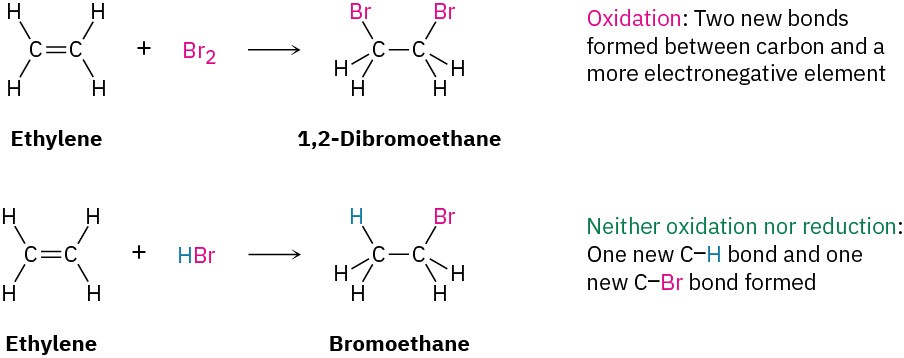
As other examples, the reaction of an alkene with Br2 to yield a 1,2-dibromide is an oxidation because two C−Br bonds are formed, but the reaction of an alkene with HBr to yield an alkyl bromide is neither an oxidation nor a reduction because both a C−H and a C−Br bond are formed.
A list of compounds of increasing oxidation level is shown in Figure 10.7. Alkanes are at the lowest oxidation level because they have the maximum possible number of C−H bonds per carbon, and CO2 is at the highest level because it has the maximum possible number of C−O bonds per carbon. Any reaction that converts a compound from a lower level to a higher level is an oxidation, any reaction that converts a compound from a higher level to a lower level is a reduction, and any reaction that doesn’t change the level is neither an oxidation nor a reduction.
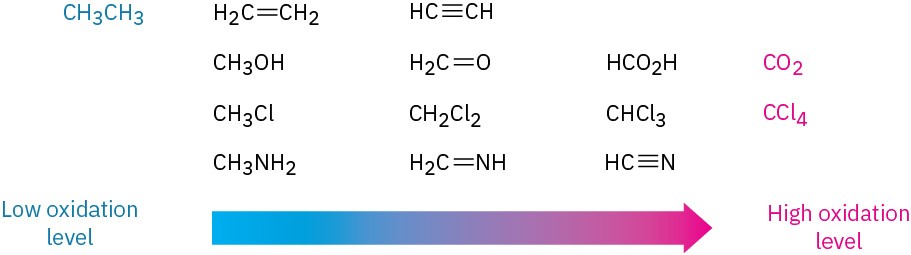
Figure 10.7 Oxidation levels of some common compounds.
Worked Example 10.2 Comparing Oxidation Levels
Rank the following compounds in order of increasing oxidation level
![]()
Strategy
Compounds that have the same number of carbon atoms can be compared by adding the number of C−O, C−N, and C−X bonds in each and then subtracting the number of C−H bonds. The larger the resultant value, the higher the oxidation level.
Solution
The first compound (propene) has six C−H bonds, giving an oxidation level of −6; the second (2-propanol) has one C−O bond and seven C−H bonds, giving an oxidation level of−6; the third (acetone) has two C−O bonds and six C−H bonds, giving an oxidation level of −4; and the fourth (propane) has eight C−H bonds, giving an oxidation level of −8. Thus, the order of increasing oxidation level is
![]()
Problem 10-12
Rank both sets of compounds in order of increasing oxidation level:
(a)
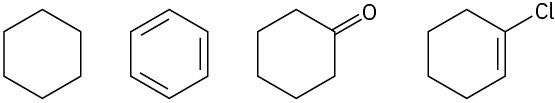
(b)
![]()
Problem 10-13
Tell whether each of the following reactions is an oxidation, a reduction, or neither.
(a)
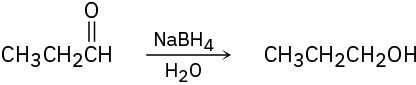
(b)