As mentioned previously, differences in chemical shifts are caused by the small local magnetic field of electrons surrounding different nuclei. Nuclei that are more strongly shielded by electrons require a higher applied field to bring them into resonance so they absorb on the right side of the NMR chart. Nuclei that are less strongly shielded need a lower applied field for resonance so they absorb on the left of the NMR chart.
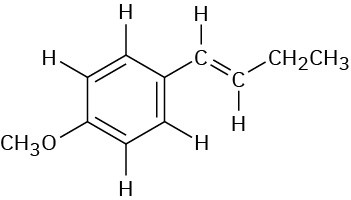
Most 1H chemical shifts fall within the range 0 to 10 δ, which can be divided into the five regions shown in Table 13.2. By remembering the positions of these regions, it’s often possible to tell at a glance what kinds of protons a molecule contains.
Table 13.2 Regions of the 1H NMR Spectrum
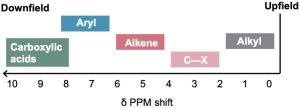
Table 13.3 shows the correlation of 1H chemical shift with electronic environment in more detail. In general, protons bonded to saturated, sp3-hybridized carbons absorb at higher fields, whereas protons bonded to sp2-hybridized carbons absorb at lower fields. Protons on carbons that are bonded to electronegative atoms, such as N, O, or halogen, also absorb at lower fields.
Table 13.3 Correlation of 1H Chemical Shifts with Environment

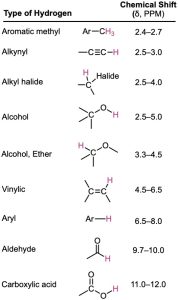
Worked Example 13.1 Predicting Chemical Shifts in 1H NMR Spectra
Methyl 2,2-dimethylpropanoate (CH3)2CCO2CH3 has two peaks in its 1H NMR spectrum. What are their approximate chemical shifts?
Strategy – Identify the types of hydrogens in the molecule, and note whether each is alkyl, vinylic, or next to an electronegative atom. Then predict where each absorbs, using Table 13.3 if necessary.
Solution – The –OCH3 protons absorb around 3.5 to 4.0 δ because they are on carbon bonded to oxygen. The (CH3)3C– protons absorb near 1.0 δ because they are typical alkane-like protons.
Problem 13-6
Each of the following compounds has a single 1H NMR peak. Approximately where would you expect each compound to absorb?
(a)
(b)
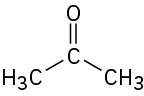
(c)
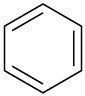
(d)
![]()
(e)
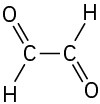
(f)
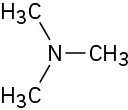
Problem 13-7
Identify the different types of protons in the following molecule, and tell where you would expect each to absorb: