NMR is used to help identify the product of nearly every reaction run in the laboratory. For example, we said in Section 8.5 that hydroboration–oxidation of alkenes occurs with anti-Markovnikov regiochemistry to yield the less highly substituted alcohol. With the help of NMR, we can now prove this statement.
Does hydroboration–oxidation of methylenecyclohexane yield cyclohexylmethanol or 1- methylcyclohexanol?
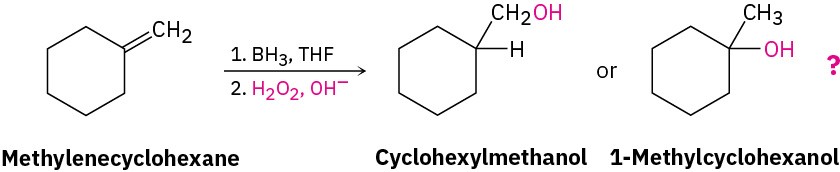
The 1H NMR spectrum of the reaction product is shown in Figure 13.16a. The spectrum shows a two-proton peak at 3.40 δ, indicating that the product has a –CH2– group bonded to an electronegative oxygen atom (–CH2OH). Furthermore, the spectrum shows no large three-proton singlet absorption near 1 δ, where we would expect the signal of a quaternary –CH3 group to appear. (Figure 13.16b) gives the spectrum of 1-methylcyclohexanol, the alternative product.) Thus, it’s clear that cyclohexylmethanol is the reaction product.
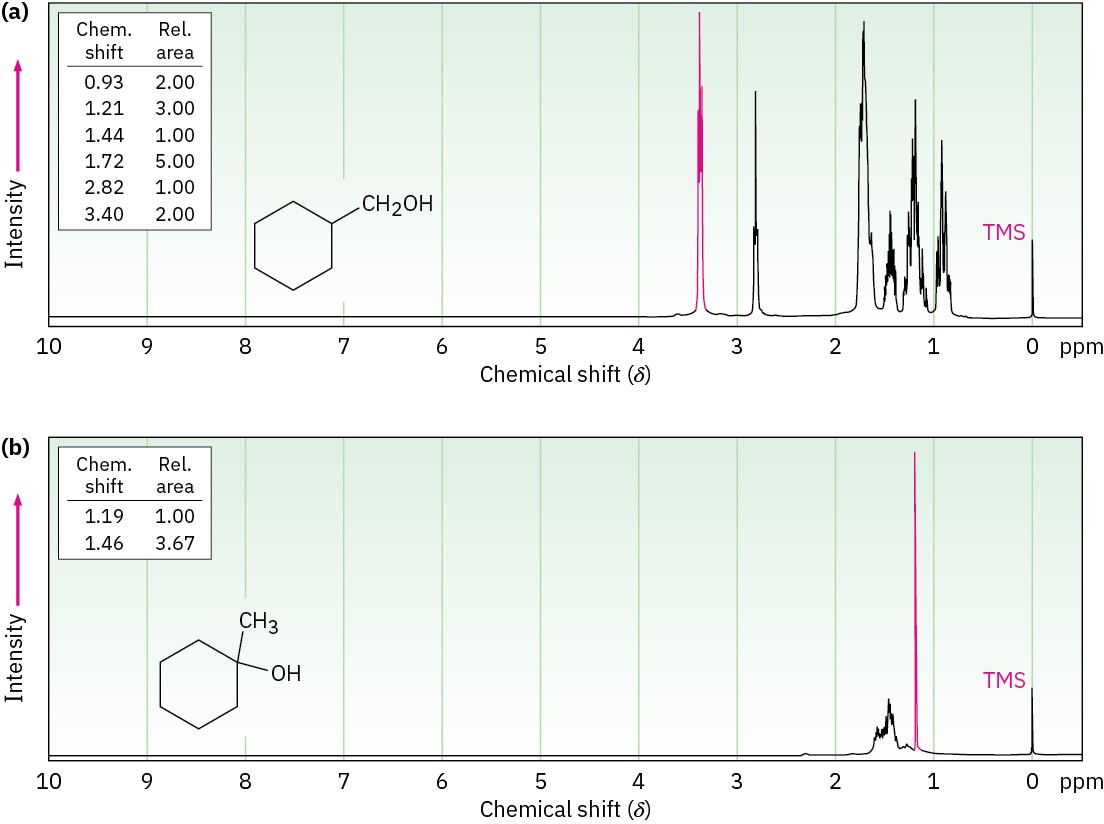
Figure 13.16 (a) The 1H NMR spectrum of cyclohexylmethanol, the product from hydroboration–oxidation of methylenecyclohexane, and (b) the 1H NMR spectrum of 1-methylcyclohexanol, the possible alternative reaction product.
Problem 13-16
How could you use 1H NMR to determine the regiochemistry of electrophilic addition to alkenes? For example, does addition of HCl to 1-methylcyclohexene yield 1-chloro-1- methylcyclohexane or 1-chloro-2-methylcyclohexane?

