A cycloaddition reaction is one in which two unsaturated molecules add to one another to yield a cyclic product. As with electrocyclic reactions, cycloadditions are governed by the orbital symmetry of the reactants. Symmetry-allowed processes often take place readily, but symmetry-disallowed processes take place with difficulty, if at all, and then only by nonconcerted pathways. Let’s look at two examples to see how they differ.
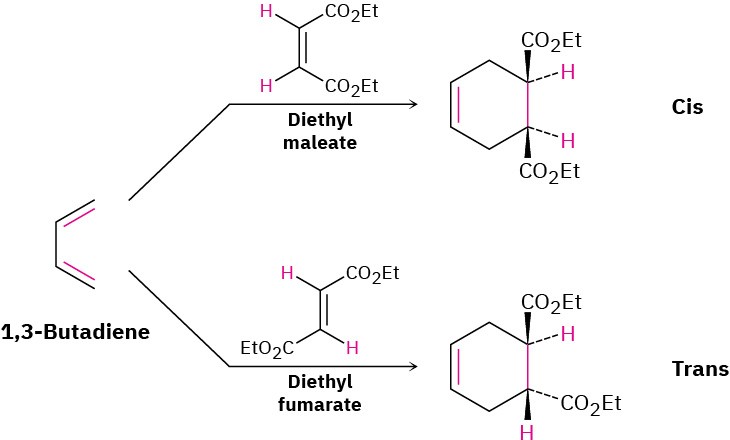
As we saw in Section 14.4, the Diels–Alder cycloaddition reaction is a pericyclic process that takes place between a diene (four π electrons) and a dienophile (two π electrons) to yield a cyclohexene product. Many thousands of Diels–Alder reactions are known. They often take place easily at room temperature or slightly above, and they are stereospecific with respect to substituents. For example, room-temperature reaction between 1,3-butadiene and diethyl maleate (cis) exclusively yields the cis-disubstituted cyclohexene product. A similar reaction between 1,3-butadiene and diethyl fumarate (trans) exclusively yields the trans-disubstituted product.
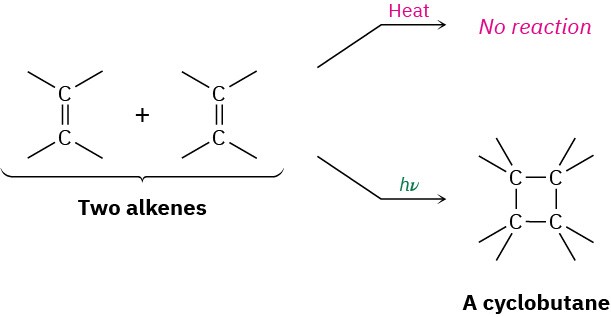
In contrast to the [4 + 2]-π-electron Diels–Alder reaction, the [2 + 2]-π-electron cycloaddition between two alkenes does not occur thermally. The [2 + 2] cycloaddition takes place only on irradiation, yielding cyclobutane products.
For a successful cycloaddition, the terminal π lobes of the two reactants must have the correct symmetry for bonding to occur. This can happen in either of two ways, called suprafacial and antarafacial. Suprafacial cycloadditions take place when a bonding interaction occurs between lobes on the same face of one reactant and lobes on the same face of the other reactant. Antarafacial cycloadditions take place when a bonding interaction occurs between lobes on the same face of one reactant and lobes on opposite faces of the other reactant (Figure 30.9).
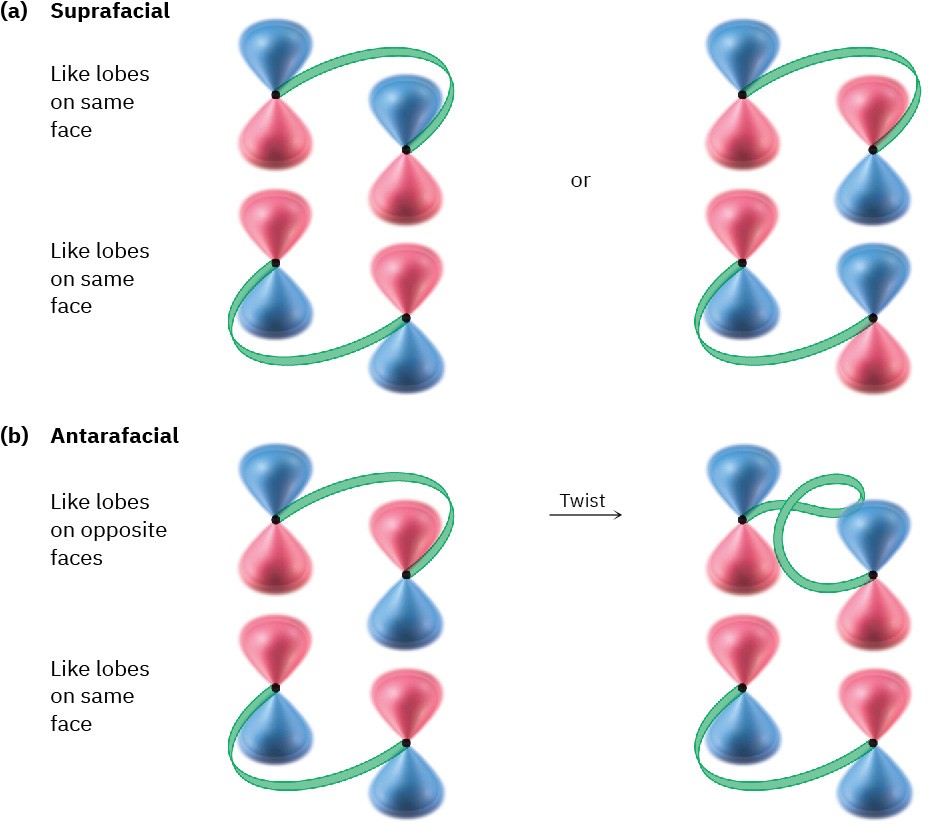
Figure 30.9
(a) Suprafacial cycloaddition occurs when there is bonding between lobes on the same face of one reactant and lobes on the same face of the other reactant.
(b) Antarafacial cycloaddition occurs when there is bonding between lobes on the same face of one reactant and lobes on opposite faces of the other, which requires a twist in one π system.
Note that both suprafacial and antarafacial cycloadditions are symmetry-allowed. Geometric constraints often make antarafacial reactions difficult, however, because there must be a twisting of the π orbital system in one of the reactants. Thus, suprafacial cycloadditions are much more common for small π systems.

