Molecules like lactic acid, alanine, and glyceraldehyde are relatively simple because each has only one stereocenter and thus only two stereoisomers. The situation becomes more complex, however, with molecules that have more than one stereocenter. As a general rule, a molecule with n stereocenters can have up to 2n stereoisomers (although it may have fewer, as we’ll see below). Take the amino acid threonine (2-amino-3- hydroxybutanoic acid), for example. Since threonine has two stereocenters (C2 and C3), there are four possible stereoisomers, as shown in Figure 5.11. Check for yourself that the R,S configurations of all stereoisomers are correct.
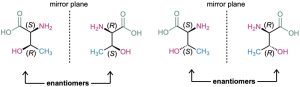
Figure 5.11 The four stereoisomers of 2-amino-3-hydroxybutanoic acid.
The four stereoisomers of 2-amino-3-hydroxybutanoic acid can be grouped into two pairs of enantiomers. The 2S,3R stereoisomer is the mirror image of 2R,3S, and the 2S,3S stereoisomer is the mirror image of 2R,3R. But what is the relationship between any two molecules that are not mirror images? What, for instance, is the relationship between the 2R,3R isomer and the 2R,3S isomer? They are stereoisomers, yet they aren’t enantiomers. To describe such a relationship, we need a new term—diastereomer.
Diastereomers are stereoisomers that are not mirror images. Note carefully the difference between enantiomers and diastereomers: enantiomers have opposite configurations at all stereocenters, whereas diastereomers have opposite configurations at some (one or more, but not all) stereocenters but the same configuration at others. A full description of the four stereoisomers of threonine is given in Table 5.2. Of the four, only the 2S,3R isomer, [α]D = −28.3, occurs naturally in plants and animals and is an essential nutrient for humans. This result is typical: most biological molecules are chiral, and usually only one stereoisomer is found in nature.
Table 5.2 Examples of stereoisomeric relationships for threonine isomers

In the special case where two diastereomers differ at only one stereocenter but are the same at all others, we say that the compounds are epimers. Cholestanol and coprostanol, for instance, are both found in human feces, and both have nine stereocenters. Eight of the nine are identical, but the one at C5 is different. Thus, cholestanol and coprostanol are epimeric at C5.

Problem 5-13
One of the following molecules (a)–(d) is D-erythrose 4-phosphate, an intermediate in the Calvin photosynthetic cycle by which plants incorporate CO2 into carbohydrates. If D-erythrose 4-phosphate has R stereochemistry at both stereocenters, which of the structures is it? Which of the remaining three structures is the enantiomer of D-erythrose 4-phosphate, and which are diastereomers?
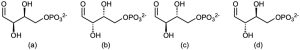
Problem 5-14
How many stereocenters does morphine have? How many stereoisomers of morphine are possible in principle?
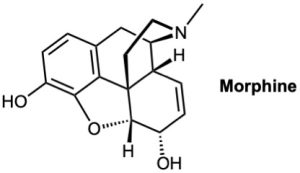
Problem 5-15
Assign R or S configuration to each stereocenter in the following molecular model of the amino acid isoleucine (blue = N):