Visualizing Chemistry
Problem 4-22
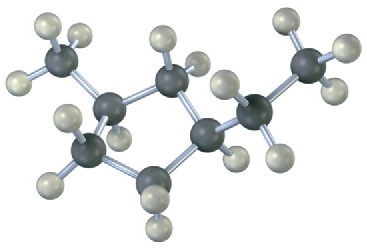
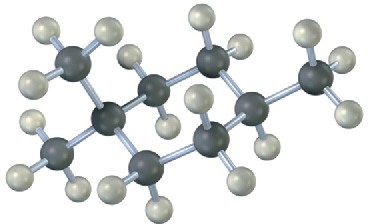
Name the following cycloalkanes:
(a)
(b)
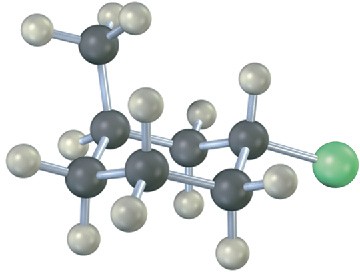
Problem 4-23
Name the following compound, identify each substituent as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (green = Cl):
Problem 4-24
A trisubstituted cyclohexane with three substituents—red, green, and blue—undergoes a ring-flip to its alternate chair conformation. Identify each substituent as axial or equatorial, and show the positions occupied by the three substituents in the ring-flipped form.
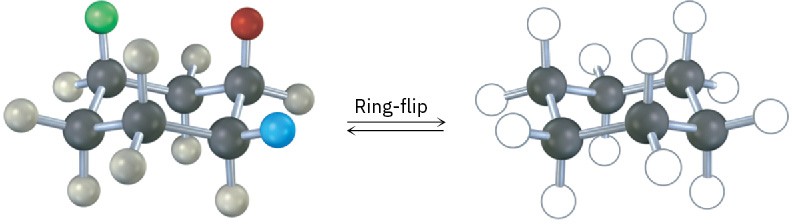
Problem 4-25
The following cyclohexane derivative has three substituents—red, green, and blue. Identify each substituent as axial or equatorial, and identify each pair of relationships (red–blue, red–green, and blue–green) as cis or trans.
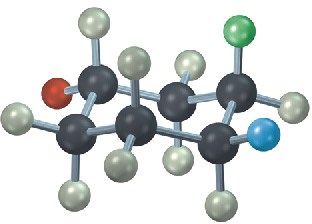
Problem 4-26
Glucose exists in two forms having a 36:64 ratio at equilibrium. Draw a skeletal structure of each, describe the difference between them, and tell which of the two you think is more stable (red = O).
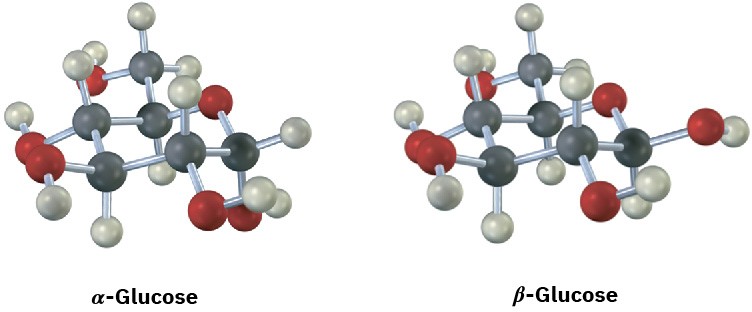
Cycloalkane Isomers
Problem 4-27
Draw the five cycloalkanes with the formula C5H10.
Problem 4-28
Give IUPAC names for the following compounds.
(a)
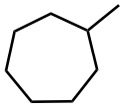
(b)
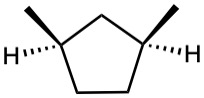
(c)
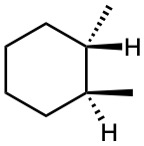
(d)
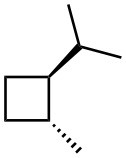
(e)
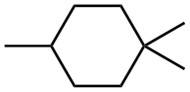
Problem 4-29
Draw a stereoisomer of trans-1,3-dimethylcyclobutane.
Problem 4-30
Tell whether the following pairs of compounds are identical, constitutional isomers, stereoisomers, or unrelated.
(a) cis-1,3-dibromocyclohexane and trans-1,4-dibromocyclohexane
(b) 2,3-dimethylhexane and 2,3,3-trimethylpentane
(c)

Problem 4-31
Draw three isomers of trans-1,2-dichlorocyclobutane, and label them as either constitutional isomers or stereoisomers.
Problem 4-32
Identify each pair of relationships among the –OH groups in glucose (red–blue, red–green, red–black, blue–green, blue–black, green–black) as cis or trans.
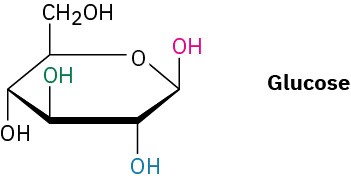
Problem 4-33
Draw 1,3,5-trimethylcyclohexane using a hexagon to represent the ring. How many cis–trans stereoisomers are possible?
Cycloalkane Conformation and Stability
Problem 4-34
Hydrocortisone, a naturally occurring hormone produced in the adrenal glands, is often used to treat inflammation, severe allergies, and numerous other conditions. Is the indicated −OH group axial or equatorial?
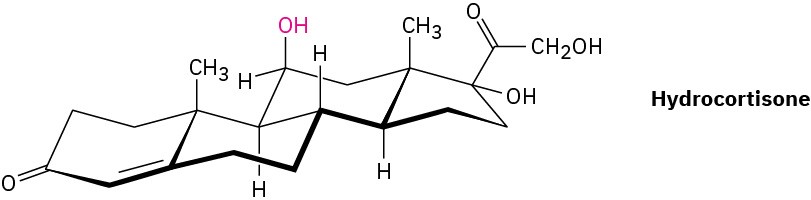
Problem 4-35
Why is a 1,3-cis disubstituted cyclohexane more stable than its trans isomer?
Problem 4-36
Which is more stable, a 1,4-trans disubstituted cyclohexane or its cis isomer?
Problem 4-37
cis-1,2-dimethylcyclobutane is less stable than its trans isomer, but cis-1,3- dimethylcyclobutane is more stable than its trans isomer. Draw the most stable conformations of both, and explain.
Cyclohexane Conformational Analysis
Problem 4-38
Draw the two chair conformations of cis-1-chloro-2-methylcyclohexane. Which is more stable?
Problem 4-39
Draw the two chair conformations of trans-1-chloro-2-methylcyclohexane. Which is more stable?
Problem 4-40
Galactose, a sugar related to glucose, contains a six-membered ring in which all the substituents except the –OH group, indicated below in red, are equatorial. Draw galactose in its more stable chair conformation.
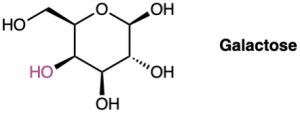
Problem 4-41
Draw the two chair conformations of menthol, and tell which is more stable.
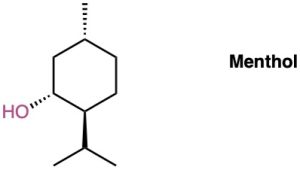
Problem 4-42
There are four cis–trans isomers of menthol (Problem 4-45), including the one shown. Draw the other three.
Problem 4-43
The diaxial conformation of cis-1,3-dimethylcyclohexane is approximately 23 kJ/mol (5.4 kcal/mol) less stable than the diequatorial conformation. Draw the two possible chair conformations, and suggest a reason for the large energy difference.
General Problems
Problem 4-44
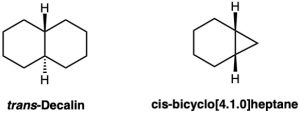
trans-decalin is more stable than its cis isomer, but cis-bicyclo[4.1.0]heptane is more stable than its trans isomer. Explain.
Problem 4-45
As mentioned in Problem 3-53, the statin drugs, such as simvastatin (Zocor), pravastatin (Pravachol), and atorvastatin (Lipitor) are the most widely prescribed drugs in the world.

(a) Are the two indicated bonds on simvastatin cis or trans?
(b) What are the cis/trans relationships among the three indicated bonds on pravastatin?
(c) Why can’t the three indicated bonds on atorvastatin be identified as cis or trans?
Problem 4-46
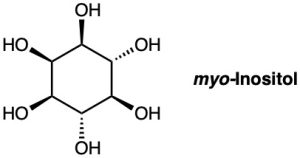
myo-inositol, one of the isomers of 1,2,3,4,5,6-hexahydroxycyclohexane, acts as a growth factor in both animals and microorganisms. Draw the most stable chair conformation of myo-inositol.
Problem 4-47
How many cis–trans stereoisomers of myo-inositol (Problem 4-55) are there? Draw the structure of the most stable isomer.
Problem 4-48
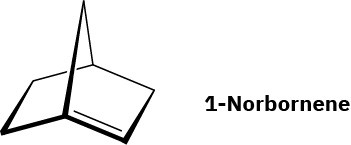
Julius Bredt, discoverer of the structure of camphor, proposed in 1935 that bicycloalkenes such as 1-norbornene, which have a double bond to a bridgehead carbon, are too strained to exist. Explain (making a molecular model will be helpful).
Problem 4-49
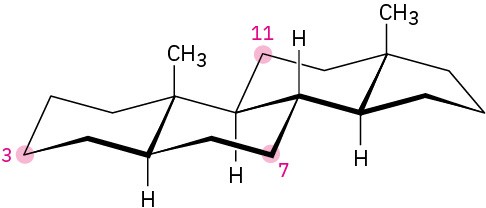
Tell whether each of the following substituents on a steroid is axial or equatorial. (A substituent that is “up” is on the top side of the molecule as drawn, and a substituent that is “down” is on the bottom side.
(a) Substituent up at C3
(b) Substituent down at C7
(c) Substituent down at C11
Problem 4-50
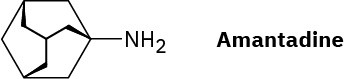
Amantadine is an antiviral agent that is active against influenza type A infection. Draw a three-dimensional representation of amantadine, showing the chair cyclohexane rings.
Problem 4-51
There are two different isomers named trans-1,2-dimethylcyclopentane. Similarly, you have two different appendages called hands. What is the relationship between them? (We’ll explore this kind of isomerism in the next chapter.)

Problem 4-52
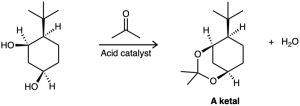
Ketones react with alcohols to yield products called ketals. Why does the all-cis isomer of 4- tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form a ketal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product ketal for each one.
Problem 4-53
Alcohols undergo an oxidation reaction to yield carbonyl compounds when treatment with CrO3. For example, 2-tert-butylcyclohexanol gives 2-tert-butylcyclohexanone. If axial −OH groups are generally more reactive than their equatorial isomers, which do you think reacts faster, the cis isomer of 2-tert-butylcyclohexanol or the trans isomer? Explain.