Additional Problems
Problem 9-14
Name the following alkynes, and predict the products of their reaction with (1) H2 in the presence of a Lindlar catalyst and (2) H3O+ in the presence of HgSO4:
(a)
(b)
Problem 9-15
From what alkyne might each of the following substances have been made? (Green = Cl.)
(a)
(b)
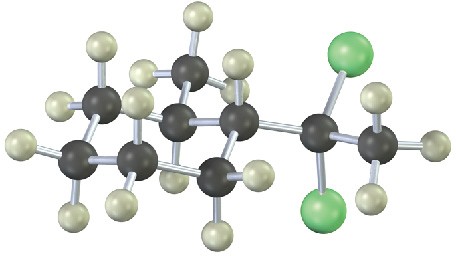
Problem 9-16
How would you prepare the following substances, starting from any compounds having four carbons or fewer?
(a)
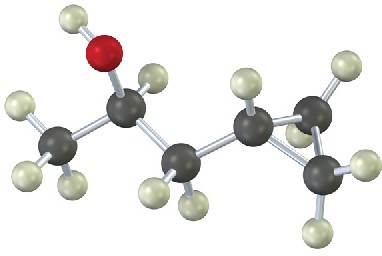
(b)
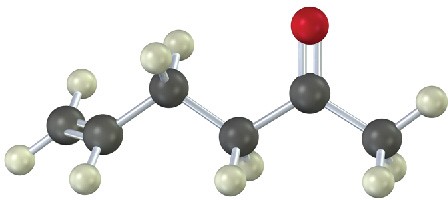
Problem 9-17
The following cycloalkyne is too unstable to exist. Explain.
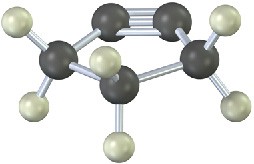
Mechanism Problems
Problem 9-18
Assuming that halogens add to alkynes in the same manner as they add to alkenes, propose a mechanism for and predict the product(s) of the reaction of phenylpropyne with Br2.
Problem 9-19
Assuming that strong acids add to alkynes in the same manner as they add to alkenes, propose a mechanism for each of the following reactions.
(a)
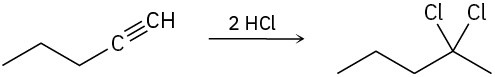
(b)
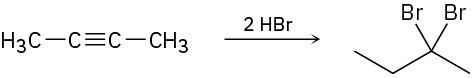
(c)
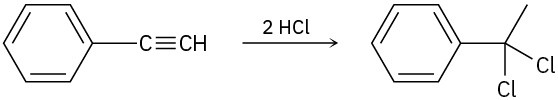
Problem 9-20
The mercury-catalyzed hydration of alkynes involves the formation of an organomercury enol intermediate. Draw the electron-pushing mechanism to show how each of the following intermediates is formed.
(a)
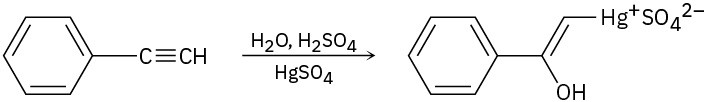
(b)
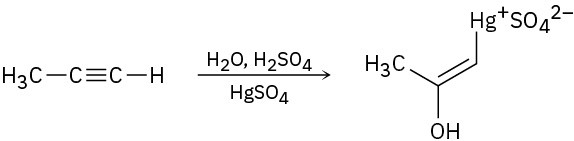
(c)
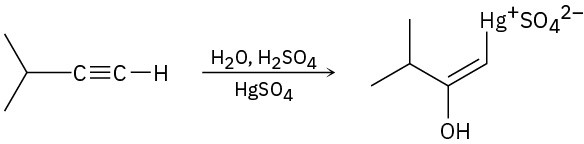
Problem 9-21
The final step in the hydration of an alkyne under acidic conditions is the tautomerization of an enol intermediate to give the corresponding ketone. The mechanism involves a protonation followed by a deprotonation. Show the mechanism for each of the following tautomerizations.
(a)
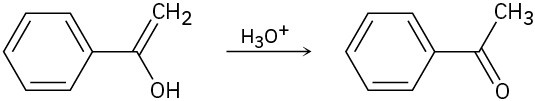
(b)
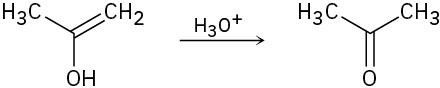
(c)
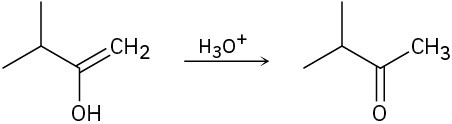
Problem 9-22
Predict the product(s) and show the complete electron-pushing mechanism for each of the following dissolving metal reductions.
(a)
![]()
(b)
![]()
(c)
![]()
Problem 9-23
Identify the mechanisms for the following reactions as polar, radical, or both.
(a)
![]()
(b)

(c)
![]()
Problem 9-24
Predict the product and provide the complete electron-pushing mechanism for the following two-step synthetic processes.
(a)
![]()
(b)
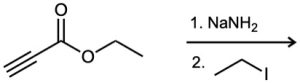
(c)
![]()
Problem 9-25
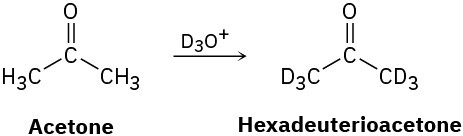
Reaction of acetone with D3O+ yields hexadeuterioacetone. That is, all the hydrogens in acetone are exchanged for deuterium. Review the mechanism of mercuric-ion-catalyzed alkyne hydration, and then propose a mechanism for this deuterium incorporation.
Naming Alkynes
Problem 9-26
Give IUPAC names for the following compounds:
(a)
![]()
(b)
![]()
(c)
![]()
(d)
![]()
(e)
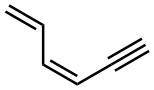
(f)
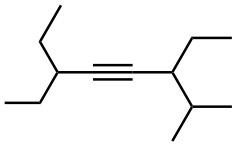
Problem 9-27
Draw structures corresponding to the following names:
(a) 3,3-Dimethyl-4-octyne
(b) 3-Ethyl-5-methyl-1,6,8-decatriyne
(c) 2,2,5,5-Tetramethyl-3-hexyne
(d) 3,4-Dimethylcyclodecyne
(e) 3,5-Heptadien-1-yne
(f) 3-Chloro-4,4-dimethyl-1-nonen-6-yne
(g) 3-sec–Butyl-1-heptyne
(h) 5-tert-Butyl-2-methyl-3-octyne
Problem 9-28
The following two hydrocarbons have been isolated from various plants in the sunflower family. Name them according to IUPAC rules.
(a) CH3CH=CHC≡CC≡CCH=CHCH=CHCH=CH2 (all trans)
(b) CH3C≡CC≡CC≡CC≡CC≡CCH=CH2
Reactions of Alkynes
Problem 9-29
Terminal alkynes react with Br2 and water to yield bromo ketones. For example:
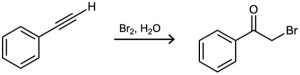
Propose a mechanism for the reaction. To what reaction of alkenes is the process analogous?
Problem 9-30
Predict the products of the following reactions:

Problem 9-31
Predict the products from reaction of 1-hexyne with the following reagents:
(a) 1 equiv HBr
(b) 1 equiv Cl2
(c) H2, Lindlar catalyst
(d) NaNH2 in NH3, then CH3Br
(e) H2O, H2SO4, HgSO4
(f) 2 equiv HCl
Problem 9-32
Predict the products from reaction of 5-decyne with the following reagents:
(a) H2, Lindlar catalyst
(b) Li in NH3
(c) 1 equiv Br2
(d) BH3 in THF, then H2O2, OH−
(e) H2O, H2SO4, HgSO4
(f) Excess H2, Pd/C catalyst
Problem 9-33
Predict the products from reaction of 2-hexyne with the following reagents:
(a) 2 equiv Br2
(b) 1 equiv HBr
(c) Excess HBr
(d) Li in NH3
(e) H2O, H2SO4, HgSO4
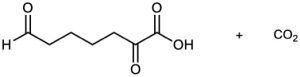
Problem 9-34
Propose structures for hydrocarbons that give the following products on oxidative cleavage by KMnO4 or O3 :
(a)
![]()
(b)
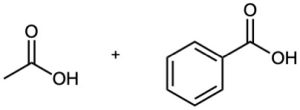
(c)
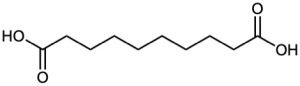
(d)
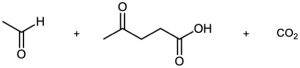
(e)
Problem 9-35
Identify the reagents a–c in the following scheme:
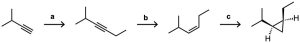
Organic Synthesis
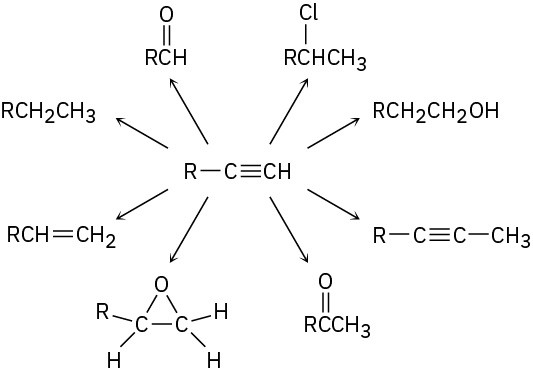
Problem 9-36
How would you carry out the following multistep conversions? More than one step may be needed in some instances.
Problem 9-37
How would you carry out the following reactions?
(a)
![]()
(b)
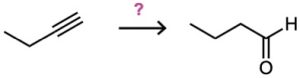
(c)
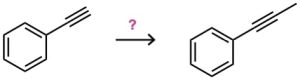
(d)

(e)
![]()
(f)
![]()
Problem 9-38
Each of the following syntheses requires more than one step. How would you carry them out?
(a)
![]()
(b)
![]()
Problem 9-39
How would you carry out the following multistep transformation?
![]()
Problem 9-40
How would you carry out the following multistep conversions?
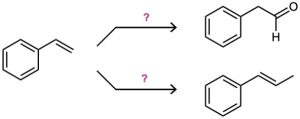
Problem 9-41
Synthesize the following compounds using 1-butyne as the only source of carbon, along with any inorganic reagents you need. More than one step may be needed.
(a) 1,1,2,2-Tetrachlorobutane
(b) 1,1-Dichloro-2-ethylcyclopropane
Problem 9-42
How would you synthesize the following compounds from acetylene and any alkyl halides with four or fewer carbons? More than one step may be needed.
(a)
![]()
(b)
![]()
(c)
![]()
(d)
![]()
(e)
![]()
Problem 9-43
How would you carry out the following reactions to introduce deuterium into organic molecules?
(a)
![]()
(b)
![]()
(c)
![]()
(d)
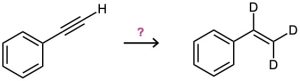
Problem 9-44
How would you prepare cyclodecyne starting from acetylene and any required alkyl halide?
Problem 9-45
The sex attractant given off by the common housefly is an alkene named muscalure. Propose a synthesis of muscalure starting from acetylene and any alkyl halides needed. What is the IUPAC name for muscalure?
![]()
General Problems
Problem 9-46
A hydrocarbon of unknown structure has the formula C8H10. On catalytic hydrogenation over the Lindlar catalyst, 1 equivalent of H2 is absorbed. On hydrogenation over a palladium catalyst, 3 equivalents of H2 are absorbed.
(a) How many degrees of unsaturation are present in the unknown structure?
(b) How many triple bonds are present?
(c) How many double bonds are present?
(d) How many rings are present?
(e) Draw a structure that fits the data.
Problem 9-47
Compound A (C9H12) absorbed 3 equivalents of H2 on catalytic reduction over a palladium catalyst to give B (C9H18). On ozonolysis, compound A gave, among other things, a ketone that was identified as cyclohexanone. On treatment with NaNH2 in NH3, followed by addition of iodomethane, compound A gave a new hydrocarbon, C (C10H14). What are the structures of A, B, and C?
Problem 9-48
Hydrocarbon A has the formula C12H8. It absorbs 8 equivalents of H2 on catalytic reduction over a palladium catalyst. On ozonolysis, only two products are formed: oxalic acid (HO2CCO2H) and succinic acid (HO2CCH2CH2CO2H). Write the reactions, and propose a structure for A.
Problem 9-49
Occasionally, a chemist might need to invert the stereochemistry of an alkene—that is, to convert a cis alkene to a trans alkene, or vice versa. There is no one-step method for doing an alkene inversion, but the transformation can be carried out by combining several reactions in the proper sequence. How would you carry out the following reactions?
(a) 
(b) 
Problem 9-50
Organometallic reagents such as sodium acetylide undergo an addition reaction with ketones, giving alcohols:
![]()
How might you use this reaction to prepare 2-methyl-1,3-butadiene, the starting material used in the manufacture of synthetic rubber?
Problem 9-51
The oral contraceptive agent Mestranol is synthesized using a carbonyl addition reaction like that shown in Problem 9-50. Draw the structure of the ketone needed.
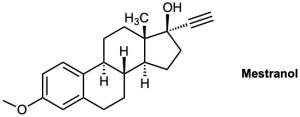
Problem 9-52
1-Octen-3-ol, a potent mosquito attractant commonly used in mosquito traps, can be prepared in two steps from hexanal, CH3CH2CH2CH2CH2CHO. The first step is an acetylide- addition reaction like that described in Problem 9-50. What is the structure of the product from the first step, and how can it be converted into 1-octen-3-ol?
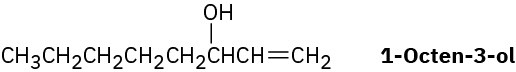
Problem 9-53
Erythrogenic acid, C18H26O2, is an acetylenic fatty acid that turns a vivid red on exposure to light. On catalytic hydrogenation over a palladium catalyst, 5 equivalents of H2 are absorbed, and stearic acid, CH3(CH2)16CO2H, is produced. Ozonolysis of erythrogenic acid gives four products: formaldehyde, CH2O; oxalic acid, HO2CCO2H; azelaic acid, HO2C(CH2)7CO2H; and the aldehyde acid OHC(CH2)4CO2H. Draw two possible structures for erythrogenic acid, and suggest a way to tell them apart by carrying out some simple reactions.
Problem 9-54
Hydrocarbon A has the formula C9H12 and absorbs 3 equivalents of H2 to yield B, C9H18, when hydrogenated over a Pd/C catalyst. On treatment of A with aqueous H2SO4 in the presence of mercury(II), two isomeric ketones, C and D, are produced. Oxidation of A with KMnO4 gives a mixture of acetic acid (CH3CO2H) and the tricarboxylic acid E. Propose structures for compounds A–D, and write the reactions.
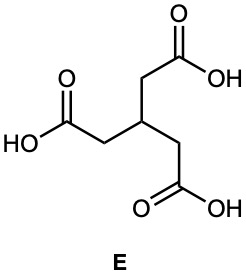
Problem 9-55
A cumulene is a compound with three adjacent double bonds. Draw an orbital picture of a cumulene. What kind of hybridization do the two central carbon atoms have? What is the geometric relationship of the substituents on one end to the substituents on the other end? What kind of isomerism is possible? Make a model to help see the answer.
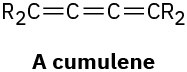
Problem 9-56
Which of the following bases could be used to deprotonate 1-butyne?
(a) KOH
(b) 
(c) 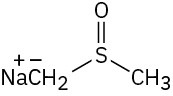
(d) 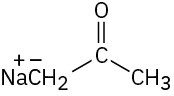
Problem 9-57
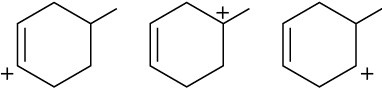
Arrange the following carbocations in order of increasing stability.
(a) 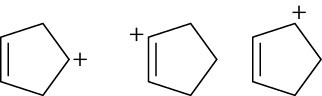
(b) 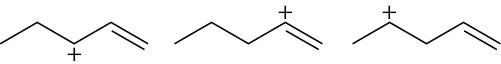
(c)