Visualizing Chemistry
Problem 13-24
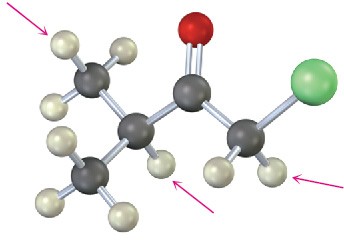
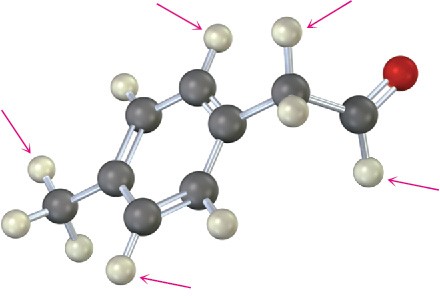
Into how many peaks would you expect the 1H NMR signals of the indicated protons to be split? (Green = Cl.)
(a)
(b)
Problem 13-25
How many absorptions would you expect the following compound to have in its 1H and 13C NMR spectra?
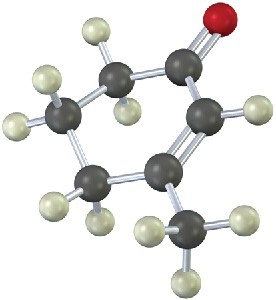
Problem 13-26
Sketch what you might expect the 1H and 13C NMR spectra of the following compound to look like (green = Cl):
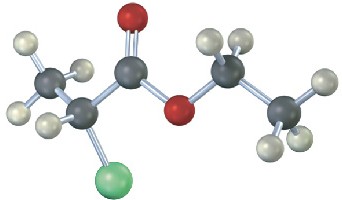
Problem 13-27
How many electronically nonequivalent kinds of protons and how many kinds of carbons are present in the following compound? Don’t forget that cyclohexane rings can ring-flip.
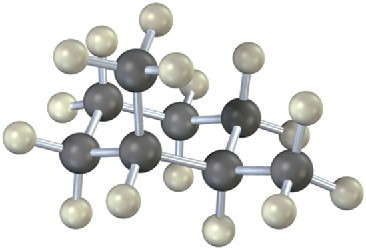
Problem 13-28
Identify the indicated protons in the following molecules as unrelated, homotopic, enantiotopic, or diastereotopic.
(a)
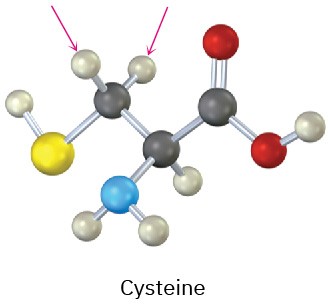
(b)
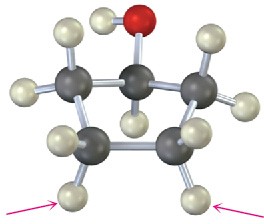
Chemical Shifts and NMR Spectroscopy
Problem 13-29
The following 1H NMR absorptions were obtained on a spectrometer operating at 200 MHz and are given in hertz downfield from the TMS standard. Convert the absorptions to δ units.
(a) 436 Hz
(b) 956 Hz
(c) 1504 Hz
Problem 13-30
The following 1H NMR absorptions were obtained on a spectrometer operating at 300 MHz. Convert the chemical shifts from δ units to hertz downfield from TMS.
(a) 2.1 δ
(b) 3.45 δ
(c) 6.30 δ
(d) 7.70 δ
Problem 13-31
When measured on a spectrometer operating at 200 MHz, chloroform (CHCl3) shows a single sharp absorption at 7.3 δ.
(a) How many parts per million downfield from TMS does chloroform absorb?
(b) How many hertz downfield from TMS would chloroform absorb if the measurement were carried out on a spectrometer operating at 360 MHz?
(c) What would be the position of the chloroform absorption in δ units when measured on a 360 MHz spectrometer?
Problem 13-32
Why do you suppose accidental overlap of signals is much more common in 1H NMR than in 13C NMR?
Problem 13-33
Is a nucleus that absorbs at 6.50 δ more shielded or less shielded than a nucleus that absorbs at 3.20 δ? Does the nucleus that absorbs at 6.50 δ require a stronger applied field or a weaker applied field to come into resonance than the one that absorbs at 3.20 δ?
1H NMR Spectroscopy
Problem 13-34
How many types of nonequivalent protons are present in each of the following molecules?
(a)
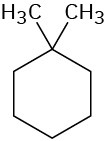
(b)
![]()
(c)
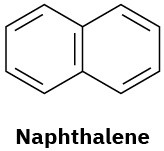
(d)
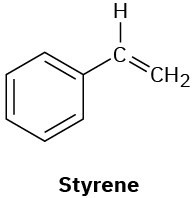
(e)
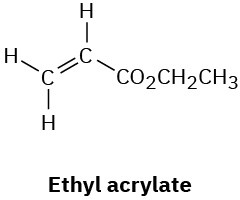
Problem 13-35
The following compounds all show a single line in their 1H NMR spectra. List them in order of expected increasing chemical shift:
CH4, CH2Cl2, cyclohexane, CH3COCH3, H2C=CH2, benzene
Problem 13-36
How many signals would you expect each of the following molecules to have in its 1H and 13C spectra?
(a)
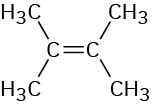
(b)
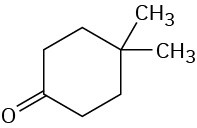
(c)
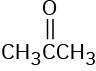
(d)
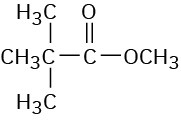
(e)
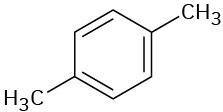
(f)
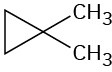
Problem 13-37
Propose structures for compounds with the following formulas that show only one peak in their 1H NMR spectra:
(a) C5H12
(b) C5H10
(c) C4H8O2
Problem 13-38
Predict the splitting pattern for each kind of hydrogen in the following molecules:
(a) (CH3)3CH
(b) CH3CH2CO2CH3
(c) trans-2-Butene
Problem 13-39
Predict the splitting pattern for each kind of hydrogen in isopropyl propanoate, CH3CH2CO2CH(CH3)2.
Problem 13-40
Identify the indicated sets of protons as unrelated, homotopic, enantiotopic, or diastereotopic:
(a)
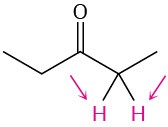
(b)
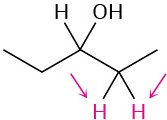
(c)
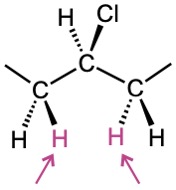
Problem 13-41
Identify the indicated sets of protons as unrelated, homotopic, enantiotopic, or diastereotopic:
(a)
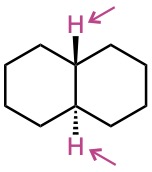
(b)
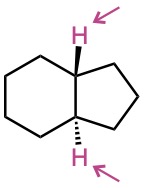
(c)
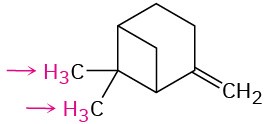
Problem 13-42
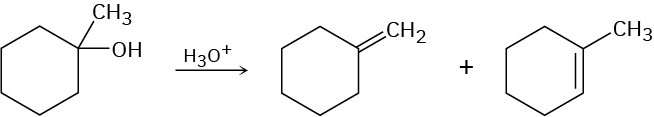
The acid-catalyzed dehydration of 1-methylcyclohexanol yields a mixture of two alkenes. How could you use 1H NMR to help you decide which was which?
Problem 13-43
How could you use 1H NMR to distinguish between the following pairs of isomers?
(a)
![]()
(b)
![]()
(c)
![]()
(d)
![]()
Problem 13-44
Propose structures for compounds that fit the following 1H NMR data:
(a) C5H10O
0.95 δ (6 H, doublet, J = 7 Hz)
2.10 δ (3 H, singlet)
2.43 δ (1 H, multiplet)
(b) C3H5Br
2.32 δ (3 H, singlet)
2.32 δ (3 H, singlet)
2.32 δ (3 H, singlet)
Problem 13-45
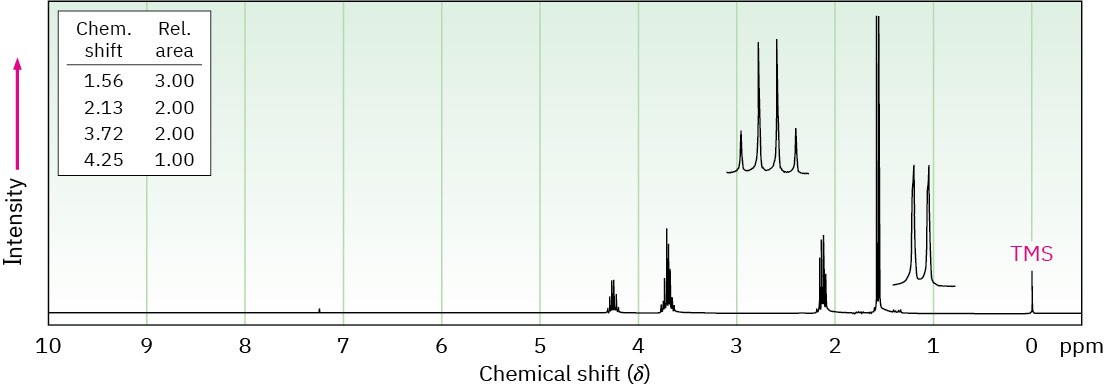
Propose structures for the two compounds whose 1H NMR spectra are shown.
(a)C4H9Br
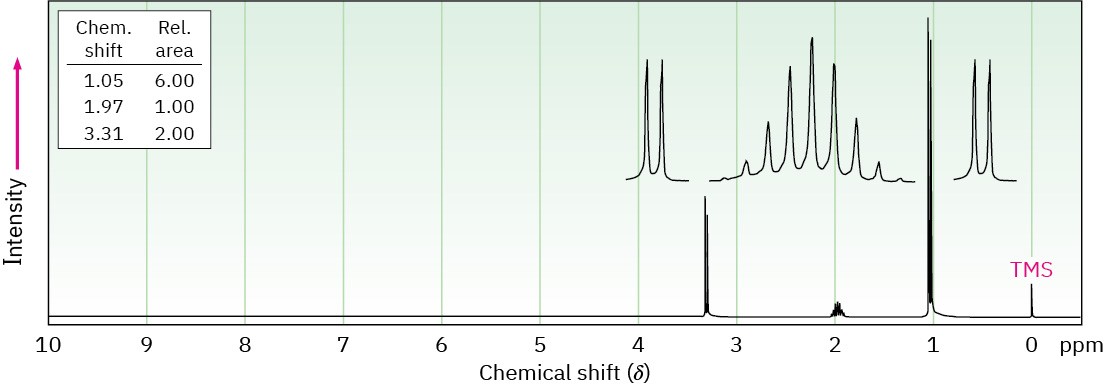
(b) C4H8Cl2
13C NMR Spectroscopy
Problem 13-46
How many 13C NMR absorptions would you expect for cis-1,3-dimethylcyclohexane? For trans-1,3-dimethylcyclohexane? Explain.
Problem 13-47
How many absorptions would you expect to observe in the 13C NMR spectra of the following compounds?
(a) 1,1-dimethylcyclohexane
(b) CH3CH2OCH3
(c) tert-butylcyclohexane
(d) 3-methyl-1-pentyne
(e) cis-1,2-dimethylcyclohexane
(f) cyclohexanone
Problem 13-48
Suppose you ran a DEPT-135 spectrum for each substance in Problem 13.47. Which carbon atoms in each molecule would show positive peaks, and which would show negative peaks?
Problem 13-49
How could you use 1H and 13C NMR to help distinguish the following isomeric compounds of the formula C4H8?
![]()
Problem 13-50
How could you use 1H NMR, 13C NMR, and IR spectroscopy to help you distinguish between the following structures?
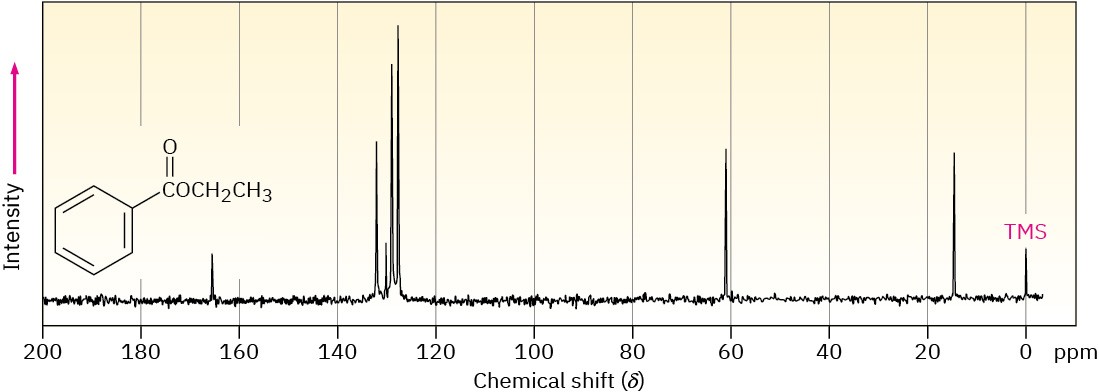
Problem 13-51
Assign as many resonances as you can to specific carbon atoms in the 13C NMR spectrum of ethyl benzoate.
General Problems
Problem 13-52
Assume that you have a compound with the formula C3H6O.
(a) How many double bonds and/or rings does your compound contain?
(b) Propose as many structures as you can that fit the molecular formula.
(c) If your compound shows an infrared absorption peak at 1715 cm–1, what functional group does it have?
(d) If your compound shows a single 1H NMR absorption peak at 2.1 δ, what is its structure?
Problem 13-53
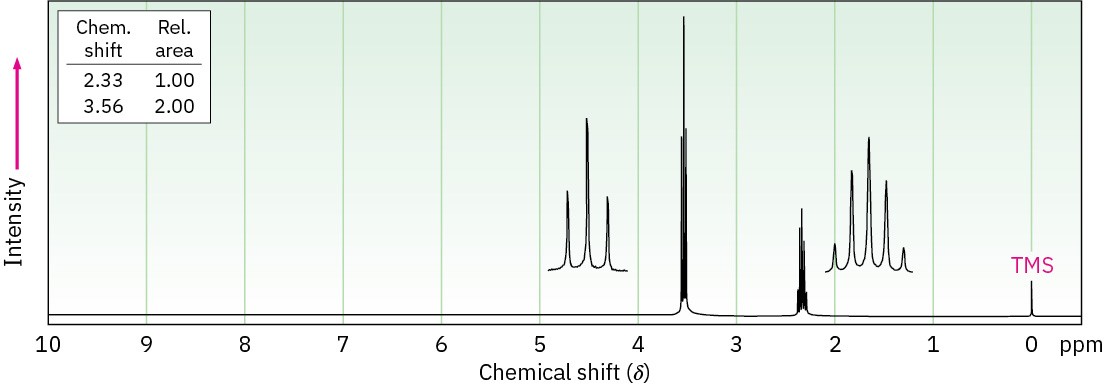
The compound whose 1H NMR spectrum is shown has the molecular formula C3H6Br2. Propose a structure.
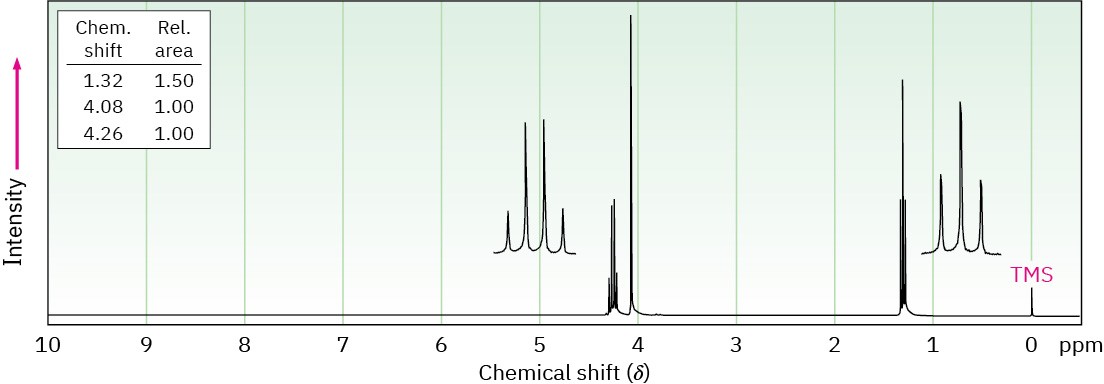
Problem 13-54
The compound whose 1H NMR spectrum is shown has the molecular formula C4H7O2Cl and has an infrared absorption peak at 1740 cm–1. Propose a structure.
Problem 13-55
Propose structures for compounds that fit the following 1H NMR data:
(a) C4H6Cl2
2.18 δ (3 H, singlet)
4.16 δ (2 H, doublet, J = 7 Hz)
5.71 δ (1 H, triplet, J = 7 Hz)
(b) C10H14
1.30 δ (9 H, singlet)
7.30 δ (5 H, singlet)
(c) C4H7BrO
2.11 δ (3 H, singlet)
3.52 δ (2 H, triplet, J = 6 Hz)
4.40 δ (2 H, triplet, J = 6 Hz)
(d) C9H11Br
2.15 δ (2 H, quintet, J = 7 Hz)
2.75 δ (2 H, triplet, J = 7 Hz)
3.38 δ (2 H, triplet, J = 7 Hz)
7.22 δ (5 H, singlet)
Problem 13-56
Long-range coupling between protons more than two carbon atoms apart is sometimes observed when π bonds intervene. An example is found in 1-methoxy-1-buten-3-yne. Not only does the acetylenic proton, Ha, couple with the vinylic proton Hb, it also couples with the vinylic proton Hc, four carbon atoms away. The data are:
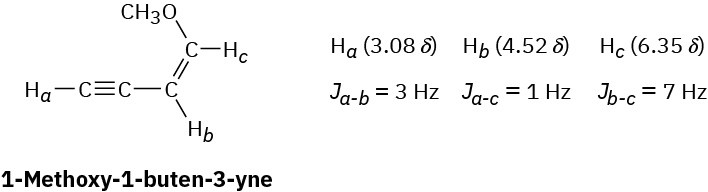
Construct tree diagrams that account for the observed splitting patterns of Ha, Hb, and Hc.
Problem 13-57
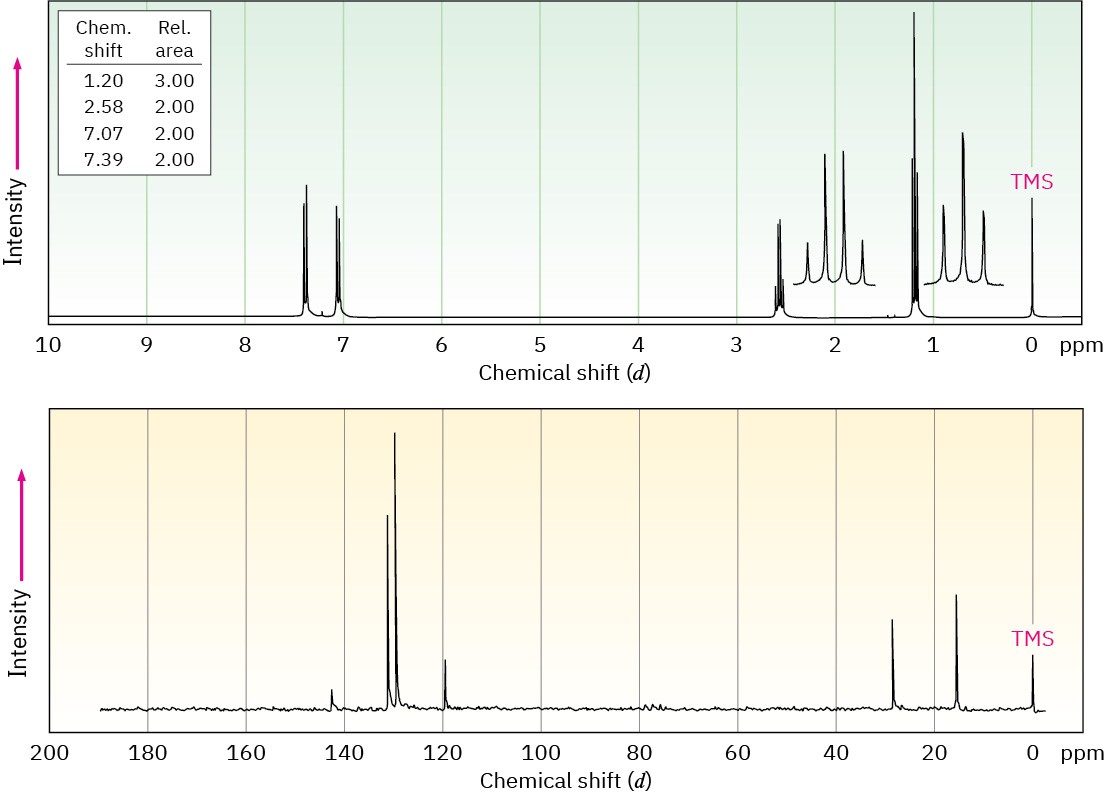
The 1H and 13C NMR spectra of compound A, C8H9Br, are shown. Propose a structure for A, and assign peaks in the spectra to your structure.
Problem 13-58
Propose structures for the three compounds whose 1H NMR spectra are shown.
(a) C5H10O
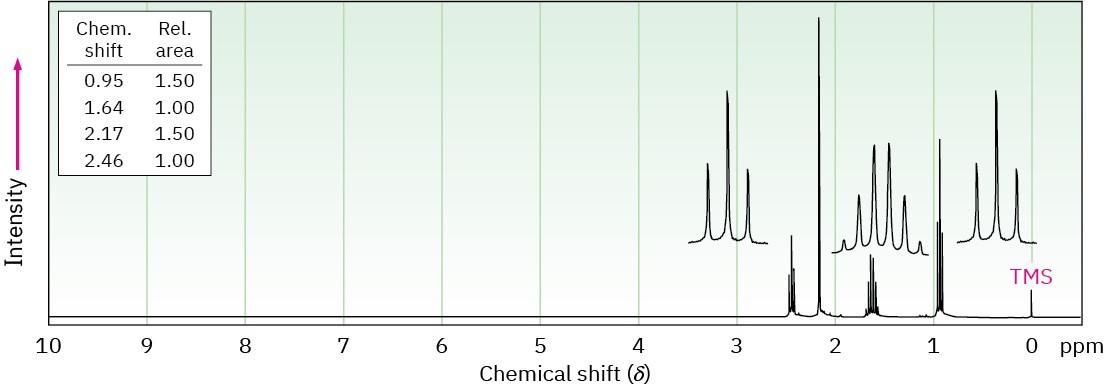
(b) C7H7Br
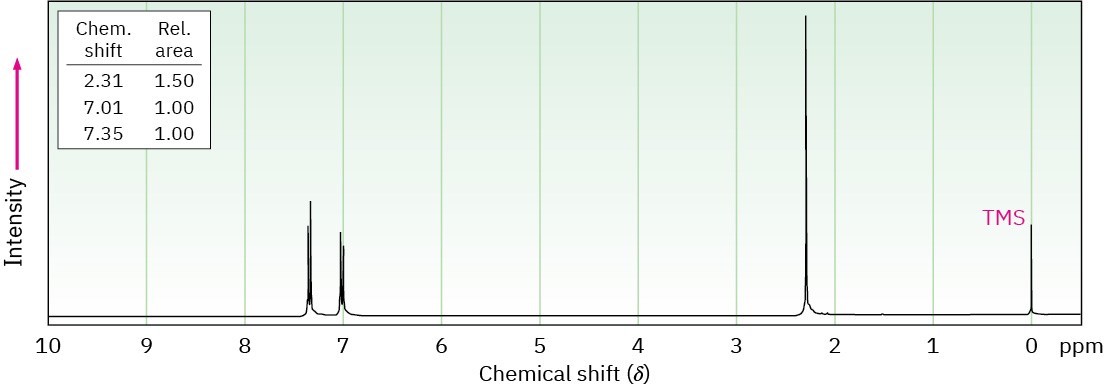
(c) C8H9Br
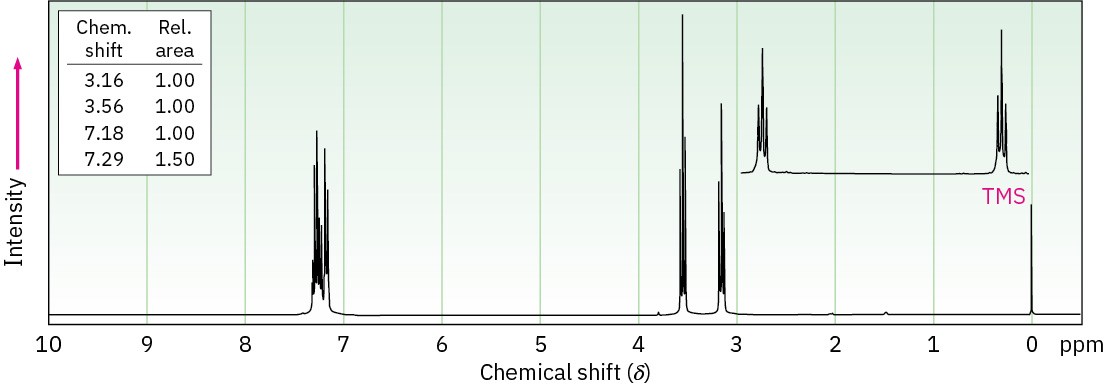
Problem 13-59
The mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this hydrocarbon, and explain the spectral data.
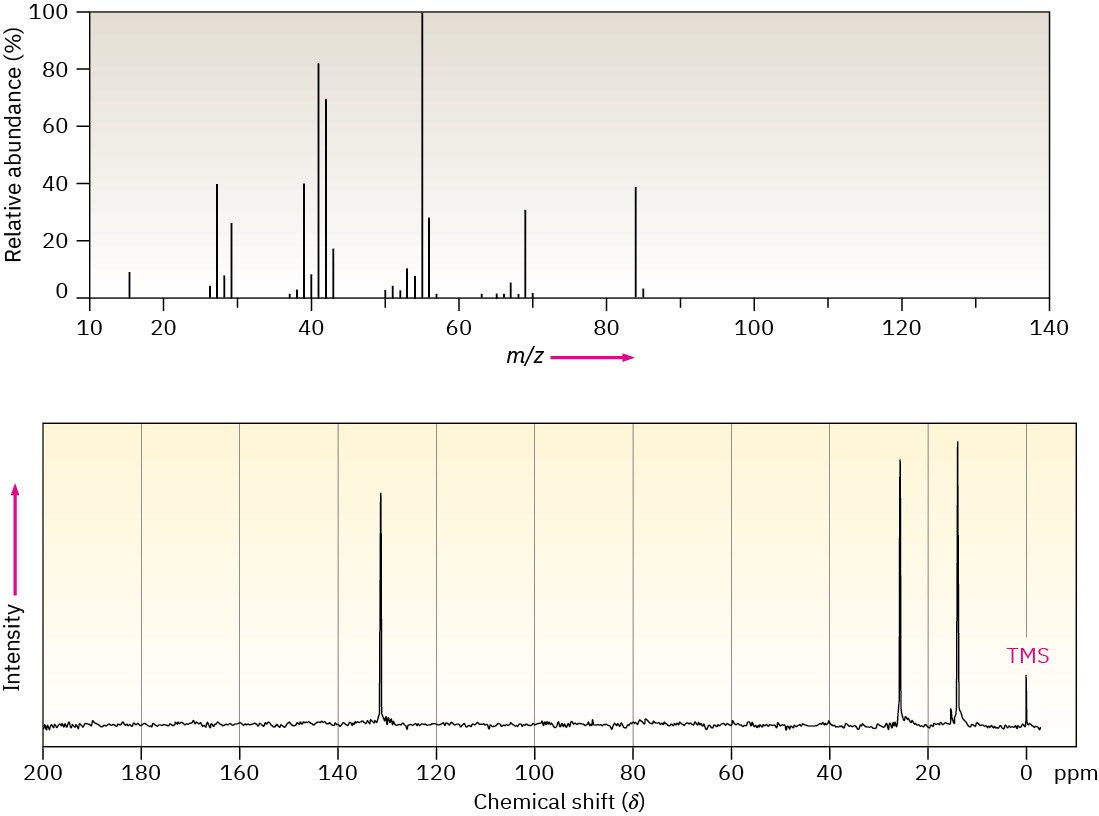
Problem 13-60
Compound A, a hydrocarbon with M+ = 96 in its mass spectrum, has the following 13C spectral data. On reaction with BH3, followed by treatment with basic H2O2, A is converted into B, whose 13C spectral data are also given. Propose structures for A and B.
Compound A
Broadband-decoupled 13C NMR: 26.8, 28.7, 35.7, 106.9, 149.7 δ
DEPT-90: no peaks
DEPT-135: no positive peaks; negative peaks at 26.8, 28.7, 35.7, 106.9 δ
Compound B
Broadband-decoupled 13C NMR: 26.1, 26.9, 29.9, 40.5, 68.2 δ
DEPT-90: 40.5 δ
DEPT-135: positive peak at 40.5 δ; negative peaks at 26.1, 26.9, 29.9, 68.2 δ
Problem 13-61
Propose a structure for compound C, which has M+ = 86 in its mass spectrum, an IR absorption at 3400 cm–1, and the following 13C NMR spectral data:
Compound C
Broadband-decoupled 13C NMR: 30.2, 31.9, 61.8, 114.7, 138.4 δ
DEPT-90: 138.4 δ
DEPT-135: positive peak at 138.4 δ; negative peaks at 30.2, 31.9, 61.8, 114.7 δ
Problem 13-62
Compound D is isomeric with compound C (Problem 13.61) and has the following 13C NMR spectral data. Propose a structure.
Compound D
Broadband-decoupled 13C NMR: 9.7, 29.9, 74.4, 114.4, 141.4 δ
DEPT-90: 74.4, 141.4 δ
DEPT-135: positive peaks at 9.7, 74.4, 141.4 δ; negative peaks at 29.9, 114.4 δ
Problem 13-63
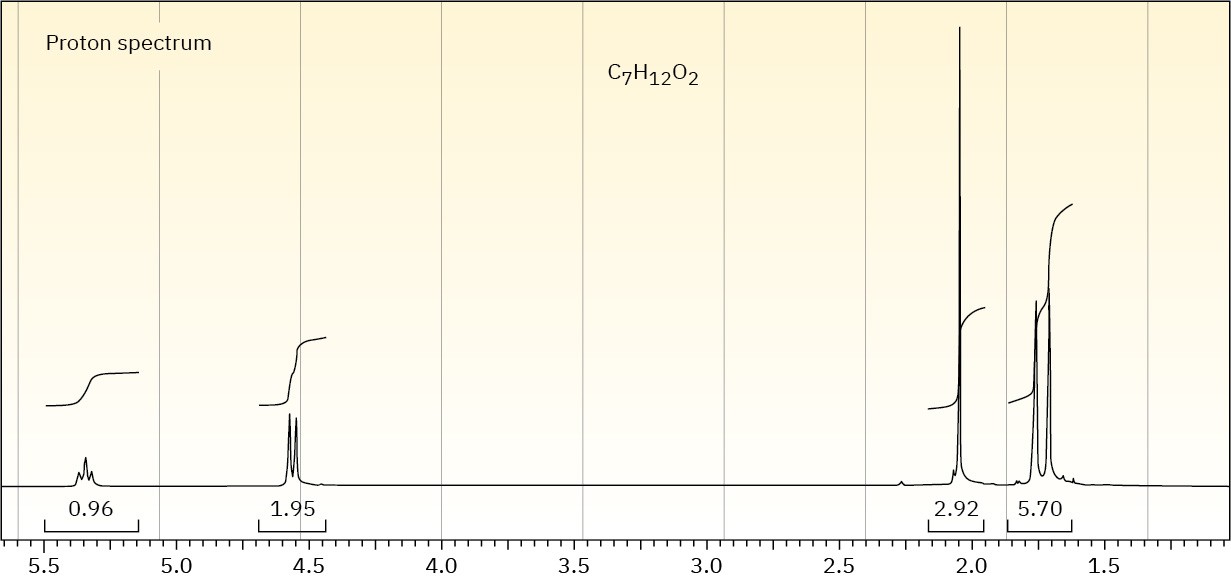
Propose a structure for compound E, C7H12O2, which has the following 13C NMR spectral data:
Compound E
Broadband-decoupled 13C NMR: 19.1, 28.0, 70.5, 129.0, 129.8, 165.8 δ
DEPT-90: 28.0, 129.8 δ
DEPT-135: positive peaks at 19.1, 28.0, 129.8 δ; negative peaks at 70.5, 129.0 δ
Problem 13-64
Compound F, a hydrocarbon with M+ = 96 in its mass spectrum, undergoes reaction with HBr to yield compound G. Propose structures for F and G, whose 13C NMR spectral data are given below.
Compound F
Broadband-decoupled 13C NMR: 27.6, 29.3, 32.2, 132.4 δ
DEPT-90: 132.4 δ
DEPT-135: positive peak at 132.4 δ; negative peaks at 27.6, 29.3, 32.2 δ
Compound G
Broadband-decoupled 13C NMR: 25.1, 27.7, 39.9, 56.0 δ
DEPT-90: 56.0 δ
DEPT-135: positive peak at 56.0 δ; negative peaks at 25.1, 27.7, 39.9 δ
Problem 13-65
3-Methyl-2-butanol has five signals in its 13C NMR spectrum at 17.90, 18.15, 20.00, 35.05, and 72.75 δ. Why are the two methyl groups attached to C3 nonequivalent? Making a molecular model should be helpful.
Problem 13-66
A 13C NMR spectrum of commercially available 2,4-pentanediol, shows five peaks at 23.3, 23.9, 46.5, 64.8, and 68.1 δ. Explain.

Problem 13-67
Carboxylic acids (RCO2H) react with alcohols (R′OH) in the presence of an acid catalyst. The reaction product of propanoic acid with methanol has the following spectroscopic properties. Propose a structure.
MS: M+ = 88 IR: 1735 cm–1
1H NMR: 1.11 δ (3 H, triplet, J = 7 Hz); 2.32 δ (2 H, quartet, J = 7 Hz); 3.65 δ (3 H, singlet)
13C NMR: 9.3, 27.6, 51.4, 174.6 δ
Problem 13-68
Nitriles (RC≡N) react with Grignard reagents (R′MgBr). The reaction product from 2- methylpropanenitrile with methylmagnesium bromide has the following spectroscopic properties. Propose a structure.
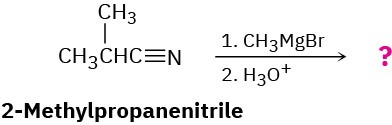
MS: M+ = 86 IR: 1715 cm–1
1H NMR: 1.05 δ (6 H, doublet, J = 7 Hz); 2.12 δ (3 H, singlet); 2.67 δ (1 H, septet, J = 7 Hz)
13C NMR: 18.2, 27.2, 41.6, 211.2 δ
Problem 13-69
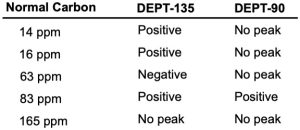
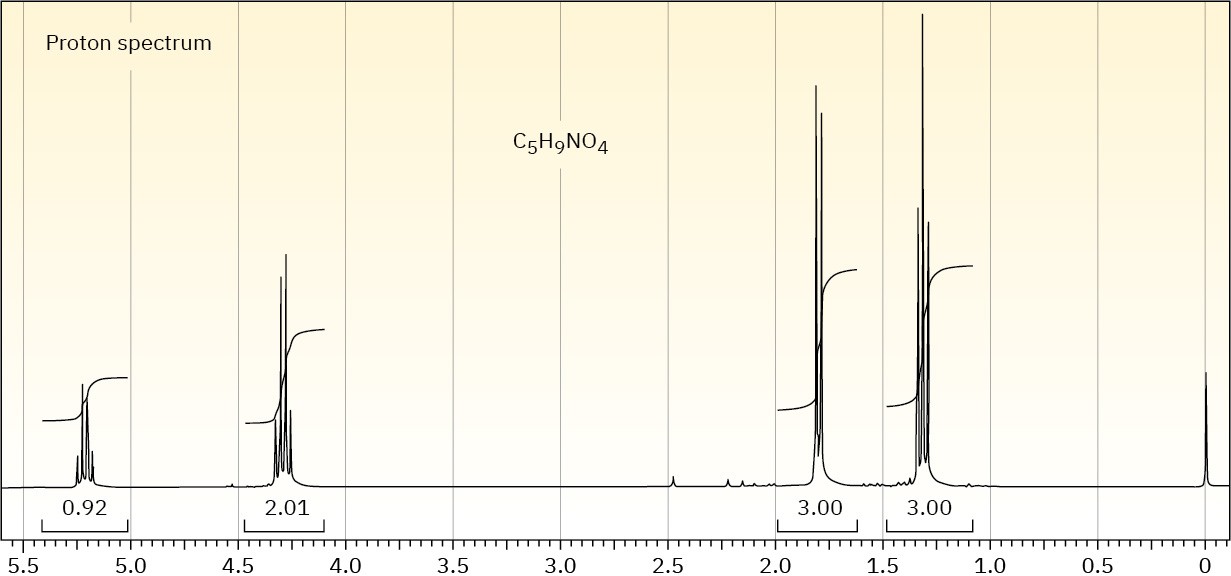
The proton NMR spectrum is shown for a compound with the formula C5H9NO4. The infrared spectrum displays strong bands at 1750 and 1562 cm–1 and a medium-intensity
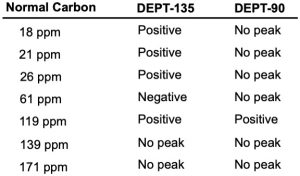
band at 1320 cm–1. The normal carbon-13 and the DEPT experimental results are tabulated. Draw the structure of this compound.
Problem 13-70
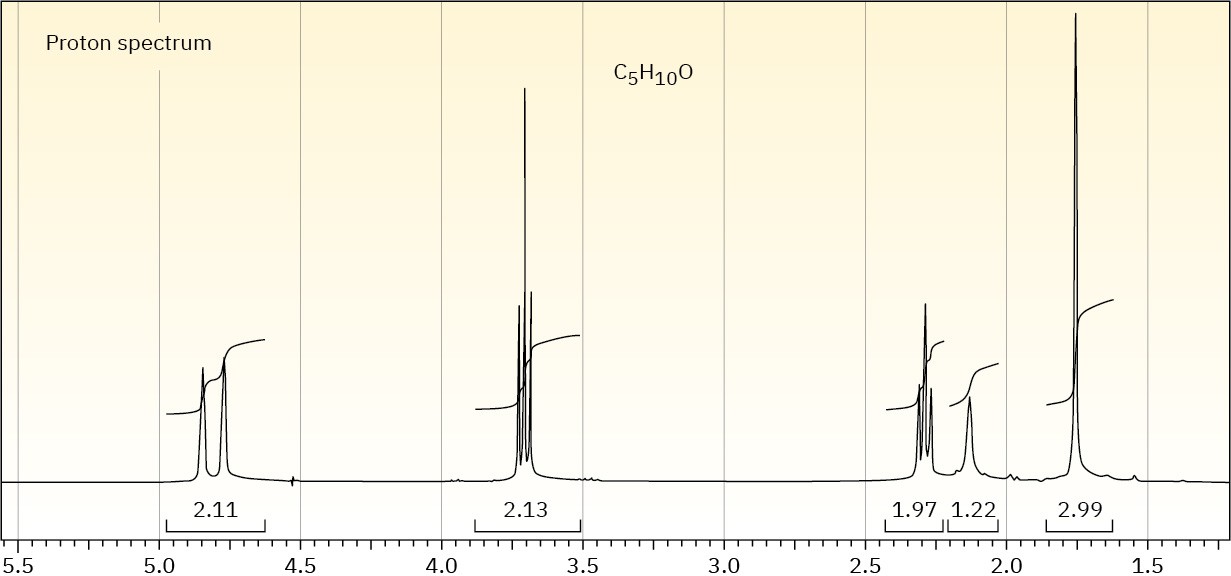
The proton NMR spectrum of a compound with the formula C5H10O is shown. The normal carbon-13 and the DEPT experimental results are tabulated. The infrared spectrum shows a broad peak at about 3340 cm–1 and a medium-sized peak at about 1651 cm–1. Draw the structure of this compound.

Problem 13-71
The proton NMR spectrum of a compound with the formula C7H12O2 is shown. The infrared spectrum displays a strong band at 1738 cm–1 and a weak band at 1689 cm–1. The normal carbon-13 and the DEPT experimental results are tabulated. Draw the structure of this compound.