Visualizing Chemistry
Problem 19-27
Each of the following substances can be prepared by a nucleophilic addition reaction between an aldehyde or ketone and a nucleophile. Identify the reactants from which each was prepared. If the substance is an acetal, identify the carbonyl compound and the alcohol; if it is an imine, identify the carbonyl compound and the amine; and so forth.
(a)
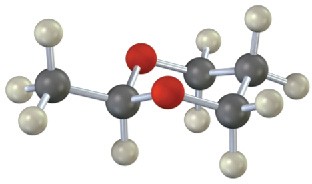
(b)
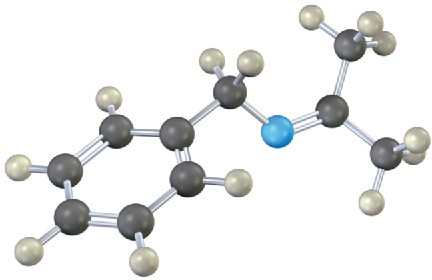
(c)
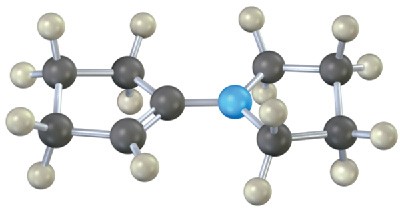
(d)
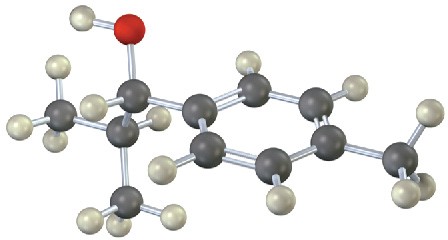
Problem 19-28
The following molecular model represents a tetrahedral intermediate resulting from addition of a nucleophile to an aldehyde or ketone. Identify the reactants, and write the structure of the final product when the nucleophilic addition reaction is complete.
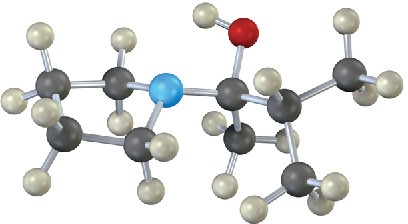
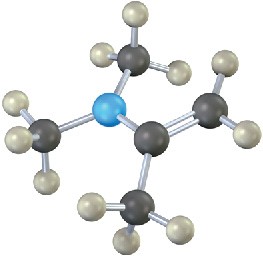
Problem 19-29
The enamine prepared from acetone and dimethylamine is shown in its lowest-energy form.
(a) What is the geometry and hybridization of the nitrogen atom?
(b) What orbital on nitrogen holds the lone pair of electrons?
(c) What is the geometric relationship between the p orbitals of the double bond and the nitrogen orbital that holds the lone pair? Why do you think this geometry represents the minimum energy?
Mechanism Problems
Problem 19-30
Predict the product(s) and propose a mechanism for each of the following reactions:
(a)
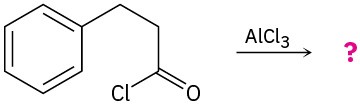
(b)
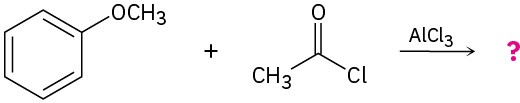
Problem 19-31
Predict the product(s) and propose a mechanism for each of the following reactions:
(a)
![]()
(b)
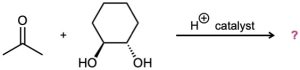
Problem 19-32
Predict the product(s) and propose a mechanism for each of the following reactions:
(a)
![]()
(b)
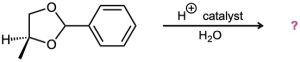
Problem 19-33
Predict the product(s) and propose a mechanism for each of the following reactions:
(a)
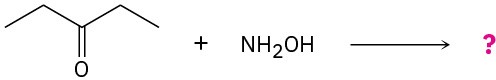
(b)
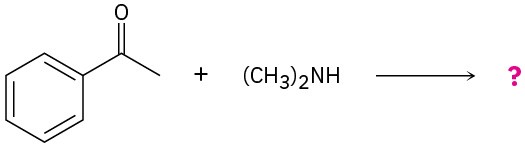
Problem 19-34
Predict the product(s) and propose mechanisms for the following reactions:
(a)
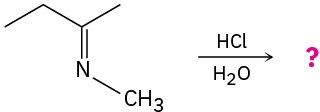
(b)
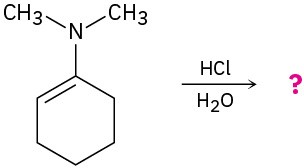
Problem 19-35
The following reaction begins with an acetal and converts it into a different acetal. Predict the product(s) and propose a mechanism.
(a)
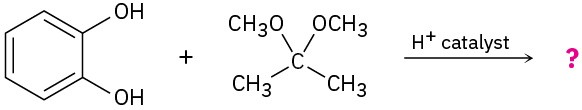
(b)
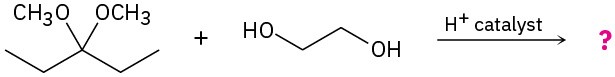
Problem 19-36
When α-glucose is treated with an acid catalyst in the presence of an alcohol, an acetal is formed. Propose a mechanism for this process and give the structure of the stereoisomeric acetal that you would also expect as a product.
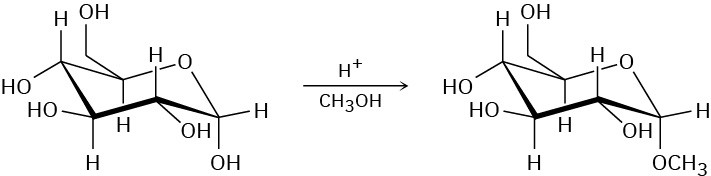
Problem 19-37
Predict the products of the following Wolff–Kishner reactions. Write the mechanism for each, beginning from the hydrazone intermediate.
(a)
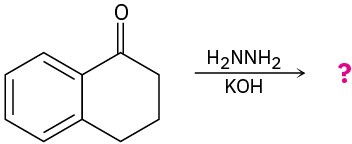
(b)
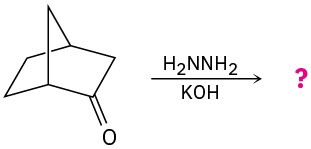
Problem 19-38
Aldehydes can be prepared by the Wittig reaction using (methoxymethylene)triphenylphosphorane as the Wittig reagent and then hydrolyzing the product with acid. For example,
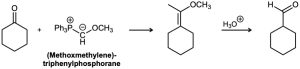
(a) How would you prepare the necessary phosphorane?
(b) Propose a mechanism for the hydrolysis step.
Problem 19-39
One of the steps in the metabolism of fats is the reaction of an unsaturated acyl CoA with water to give a β-hydroxyacyl CoA. Propose a mechanism.
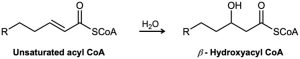
Problem 19-40
Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:
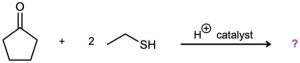
Problem 19-41
Ketones react with dimethylsulfonium methylide to yield epoxides. Suggest a mechanism for the reaction.
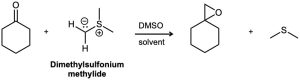
Problem 19-42
Propose a mechanism for the following reaction.
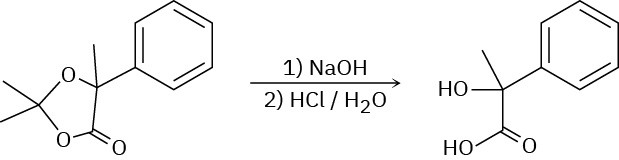
Problem 19-43
Paraldehyde, a sedative and hypnotic agent, is prepared by treatment of acetaldehyde with an acidic catalyst. Propose a mechanism for the reaction.
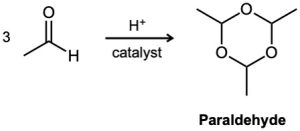
Problem 19-44
The Meerwein–Ponndorf–Verley reaction involves reduction of a ketone by treatment with an excess of aluminum triisopropoxide, [(CH3)2CHO]3Al. The mechanism of the process is closely related to the Cannizzaro reaction in that a hydride ion acts as a leaving group.
Propose a mechanism.
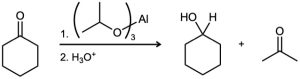
Problem 19-45
Propose a mechanism to account for the formation of 3,5-dimethylpyrazole from hydrazine and 2,4-pentanedione. What has happened to each carbonyl carbon in going from starting material to product.
Problem 19-46
In light of your answer to Problem 19-45, propose a mechanism for the formation of 3,5-dimethylisoxazole from hydroxylamine and 2,4-pentanedione.
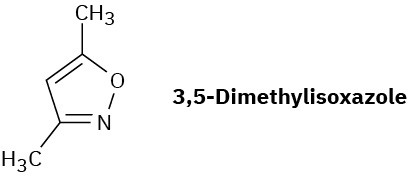
Problem 19-47
Trans alkenes are converted into their cis isomers and vice versa on epoxidation followed by treatment of the epoxide with triphenylphosphine. Propose a mechanism for the reaction.
![]()
Problem 19-48
Treatment of an α,β-unsaturated ketone with basic aqueous hydrogen peroxide yields an epoxy ketone. The reaction is specific to unsaturated ketones; isolated alkene double bonds do not react. Propose a mechanism.
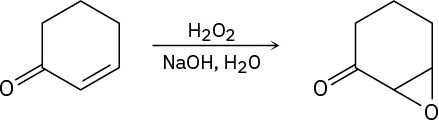
Problem 19-49
One of the biological pathways by which an amine is converted to a ketone involves two steps: (1) oxidation of the amine by NAD+ to give an imine and (2) hydrolysis of the imine to give a ketone plus ammonia. Glutamate, for instance, is converted by this process into α-ketoglutarate. Show the structure of the imine intermediate, and propose mechanisms for both steps.
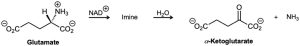
Problem 19-50
Primary amines react with esters to yield amides: RCO2R′ + R″NH2 → RCONHR″ + R′OH. Propose a mechanism for the following reaction of an α,β-unsaturated ester.
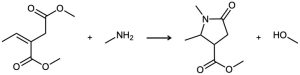
Problem 19-51
When crystals of pure α-glucose are dissolved in water, isomerization occurs slowly to produce β-glucose. Propose a mechanism for the isomerization.
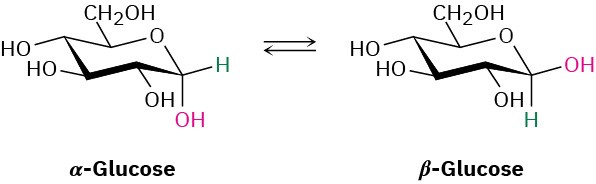
Problem 19-52
The Wharton reaction converts an epoxy ketone to an allylic alcohol by reaction with hydrazine. Review the Wolff–Kishner reaction in Section 19.9 and then propose a mechanism.
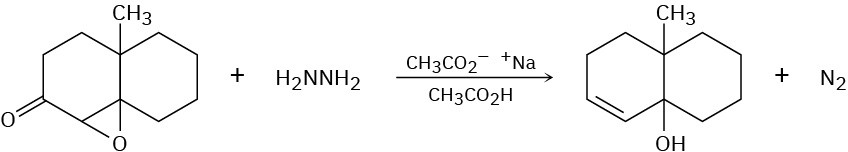
Naming Aldehydes and Ketones
Problem 19-53
Draw structures corresponding to the following names:
(a) bromoacetone
(b) (S)-2-hydroxypropanal
(c) 2-methyl-3-heptanone
(d) (2S,3R)-2,3,4-trihydroxybutanal
(e) 2,2,4,4-tetramethyl-3-pentanone
(f) 4-methyl-3-penten-2-one
(g) butanedial
(h) 3-phenyl-2-propenal
(i) 6,6-dimethyl-2,4-cyclohexadienone
(j) p-nitroacetophenone
Problem 19-54
Draw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?
Problem 19-55
Give IUPAC names for the following compounds:
(a)
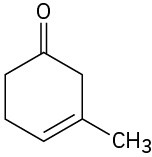
(b)

(c)
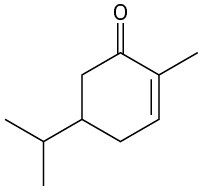
(d)

(e)
![]()
(f)
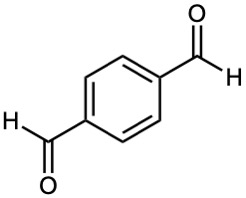
Problem 19-56
Draw structures of compounds that fit the following descriptions:
(a) an α,β-unsaturated ketone, C6H8O
(b) an α-diketone
(c) an aromatic ketone, C9H10O
(d) a diene aldehyde, C7H8O
Reactions of Aldehydes and Ketones
Problem 19-57
Predict the products of the reaction of (1) phenylacetaldehyde and (2) acetophenone with the following reagents:
(a) NaBH4, then H3O+
(b) Dess–Martin reagent
(c) NH2OH, HCl catalyst
(d) CH3MgBr, then H3O+
(e) 2 CH3OH, HCl catalyst
(f) H2NNH2, KOH
(g) (C6H5)3P=CH2
(h) HCN, KCN
Problem 19-58
Show how you might use a Wittig reaction to prepare the following alkenes. Identify the alkyl halide and the carbonyl components.
(a)
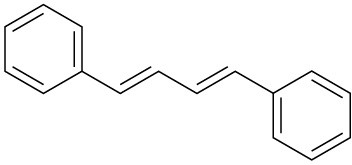
(b)
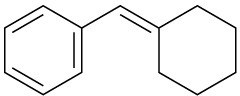
Problem 19-59
How would you use a Grignard reaction on an aldehyde or ketone to synthesize the following compounds?
(a) 2-pentanol
(b) 1-butanol
(c) 1-phenylcyclohexanol
(d) diphenylmethanol
Problem 19-60
How might you carry out the following selective transformations? One of the two schemes requires a protection step. (Recall from Section 19.4 that aldehydes are more reactive than ketones toward nucleophilic addition.)
(a)
![]()
(b)
![]()
Problem 19-61
How would you prepare the following substances from 2-cyclohexenone? More than one step may be needed.
(a)
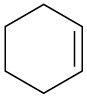
(b)
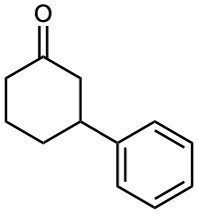
(c)
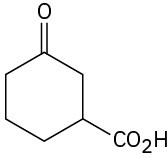
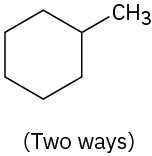
Problem 19-62
How would you synthesize the following substances from benzaldehyde and any other reagents needed?
(a)
![]()
(b)
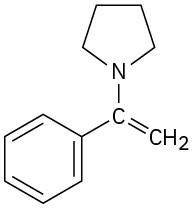
(c)
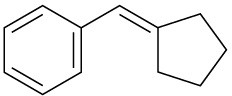
Problem 19-63
Carvone is the major constituent of spearmint oil. What products would you expect from reaction of carvone with the following reagents?
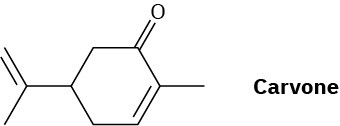
(a) (CH3)2Cu– Li+, then H3O+
(b) LiAlH4, then H3O+
(c) CH3NH2
(d)C6H5MgBr, then H3O+
(e) H2/Pd
(f) HOCH2CH2OH, HCl
(g) (C6H5)3PCHCH3
Problem 19-64
How would you synthesize the following compounds from cyclohexanone?
(a) methylcyclohexene
(b) phenylcyclohexanone
(c)cis-1,2-cyclohexanediol
(d) 1-cyclohexylcyclohexanol
Spectroscopy
Problem 19-65
At what position would you expect to observe IR absorptions for the following molecules?
(a)
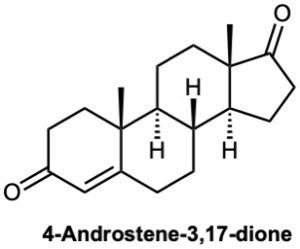
(b)
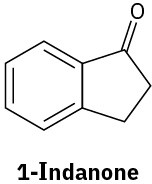
(c)
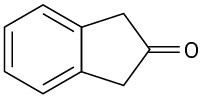
(d)
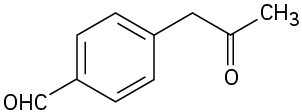
Problem 19-66
Acid-catalyzed dehydration of 3-hydroxy-3-phenylcyclohexanone leads to an unsaturated ketone. What possible structures are there for the product? At what position in the IR spectrum would you expect each to absorb? If the actual product has an absorption at 1670 cm–1, what is its structure?
Problem 19-67
Choose the structure that best fits the IR spectrum shown.
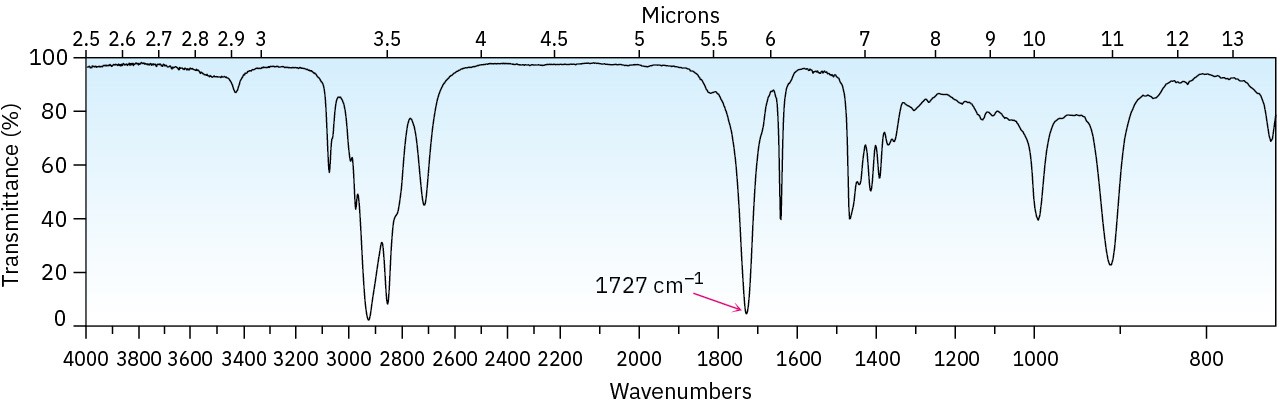

Problem 19-68
Propose structures for molecules that meet the following descriptions. Assume that the kinds of carbons (1°, 2°, 3°, or 4°) have been assigned by DEPT–NMR.
(a) C6H12O; IR: 1715 cm–1; 13C NMR: 8.0 δ (1°), 18.5 δ (1°), 33.5 δ (2°), 40.6 δ (3°), 214.0 δ (4°)
(b) C5H10O; IR: 1730 cm–1; 13C NMR: 22.6 δ (1°), 23.6 δ (3°), 52.8 δ (2°), 202.4 δ (3°)
(c) C6H8O; IR: 1680 cm–1; 13C NMR: 22.9 δ (2°), 25.8 δ (2°), 38.2 δ (2°), 129.8 δ (3°), 150.6 δ (3°), 198.7 δ (4°)
Problem 19-69
Compound A, C8H10O2, has an intense IR absorption at 1750 cm–1 and gives the 13C NMR spectrum shown. Propose a structure for A.
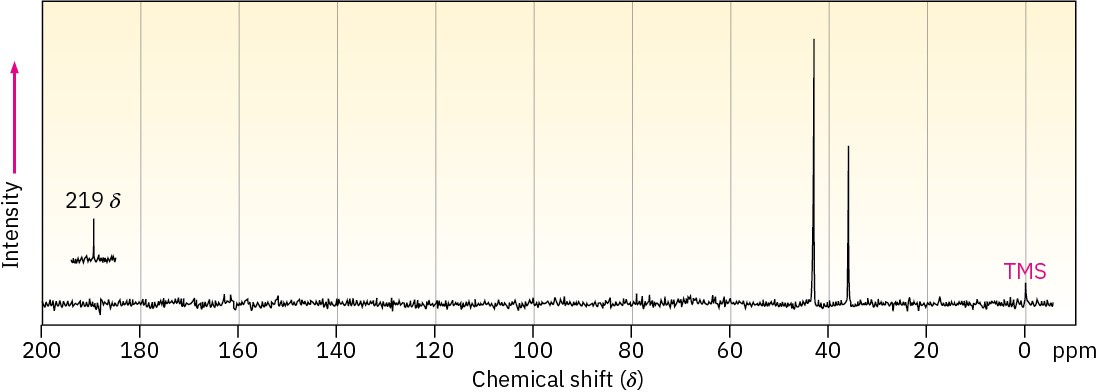
Problem 19-70
Propose structures for ketones or aldehydes that have the following 1H NMR spectra:
(a) C4H7ClO
IR: 1715 cm–1
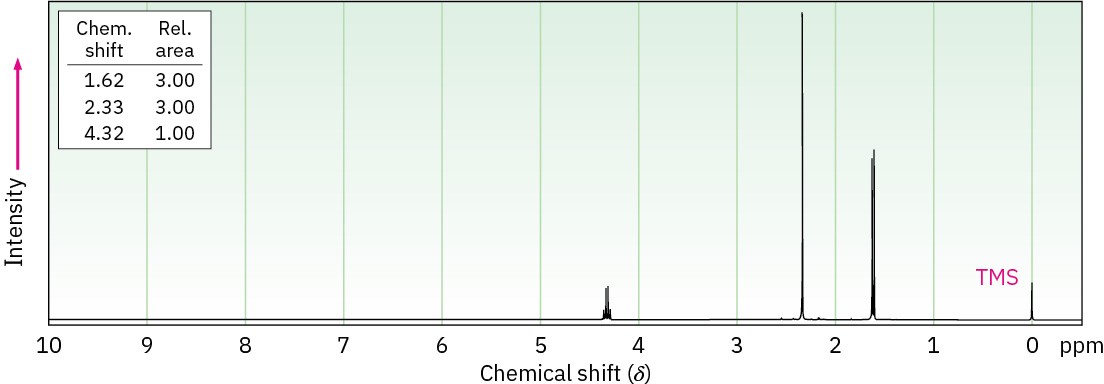
(b) C7H14O
IR: 1710 cm–1
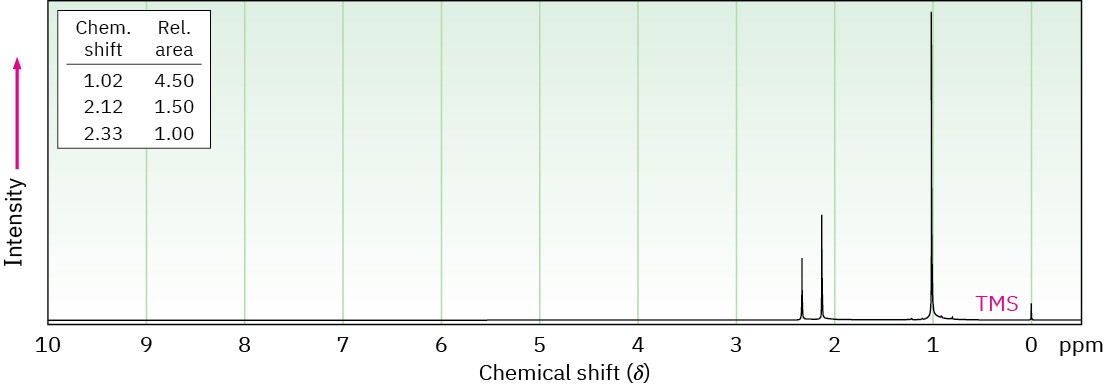
General Problems
Problem 19-71
When 4-hydroxybutanal is treated with methanol in the presence of an acid catalyst, 2-methoxytetrahydrofuran is formed. Explain.
![]()
Problem 19-72
The SN2 reaction of (dibromomethyl)benzene, C6H5CHBr2, with NaOH yields benzaldehyde rather than (dihydroxymethyl)benzene, C6H5CH(OH)2. Explain.
Problem 19-73
Reaction of 2-butanone with HCN yields a chiral product. What stereochemistry does the product have? Is it optically active?
Problem 19-74
The amino acid methionine is biosynthesized by a multistep route that includes reaction of an imine of pyridoxal phosphate (PLP) to give an unsaturated imine, which then reacts with cysteine. What kinds of reactions are occurring in the two steps?
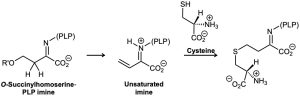
Problem 19-75
Each of the following reaction schemes has one or more flaws. What is wrong in each case? How would you correct each scheme?
(a)
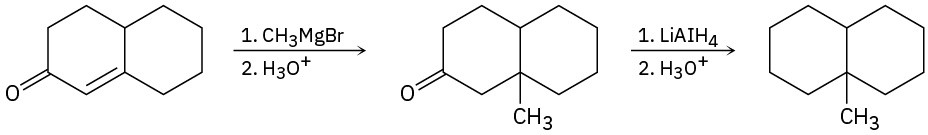
(b)
![]()
(c)
![]()
Problem 19-76
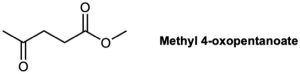
6-Methyl-5-hepten-2-one is a constituent of lemongrass oil. How could you synthesize this substance from methyl 4-oxopentanoate?
Problem 19-77
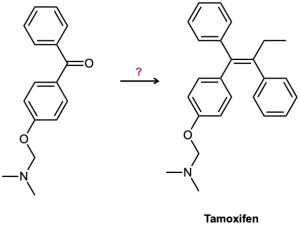
Tamoxifen is a drug used in the treatment of breast cancer. How would you prepare tamoxifen from benzene, the following ketone, and any other reagents needed?
Problem 19-78
Compound A, MW = 86, shows an IR absorption at 1730 cm–1 and a very simple 1H NMR spectrum with peaks at 9.7 δ (1 H, singlet) and 1.2 δ (9 H, singlet). Propose a structure for A.
Problem 19-79
Compound B is isomeric with A (Problem 19-78) and shows an IR peak at 1715 cm–1. The 1H NMR spectrum of B has peaks at 2.4 δ (1 H, septet, J = 7 Hz), 2.1 δ (3 H, singlet), and 1.2 δ (6 H, doublet, J = 7 Hz). What is the structure of B?
Problem 19-80
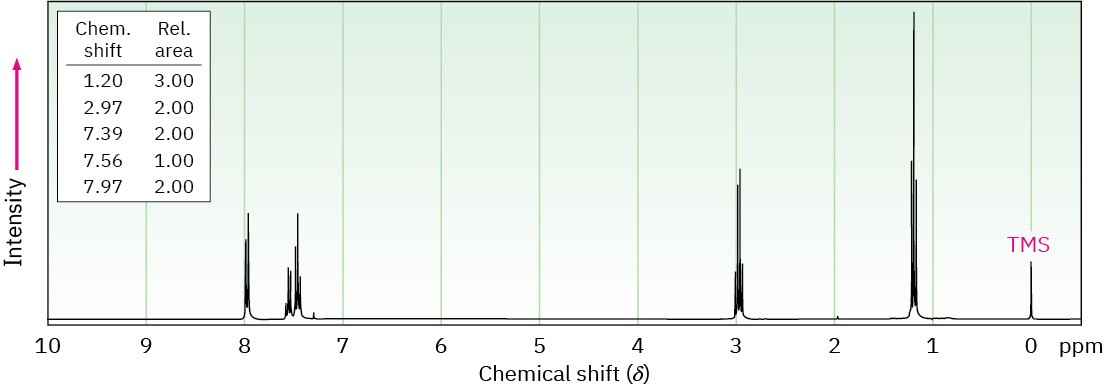
The 1H NMR spectrum shown is that of a compound with the formula C9H10O. How many double bonds and/or rings does this compound contain? If the unknown compound has an IR absorption at 1690 cm–1, what is a likely structure?
Problem 19-81
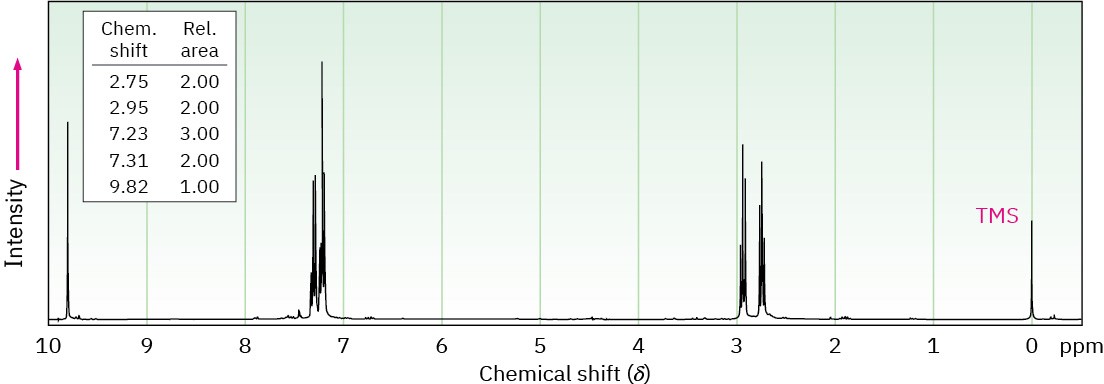
The 1H NMR spectrum shown is that of a compound isomeric with the one in Problem 19-80. This isomer has an IR absorption at 1730 cm–1. Propose a structure. [Note: Aldehyde protons (CHO) often show low coupling constants to adjacent hydrogens, so the splitting of aldehyde signals is not always apparent.]
Problem 19-82
Propose structures for ketones or aldehydes that have the following 1H NMR spectra:
(a) C9H10O2
IR: 1695 cm–1
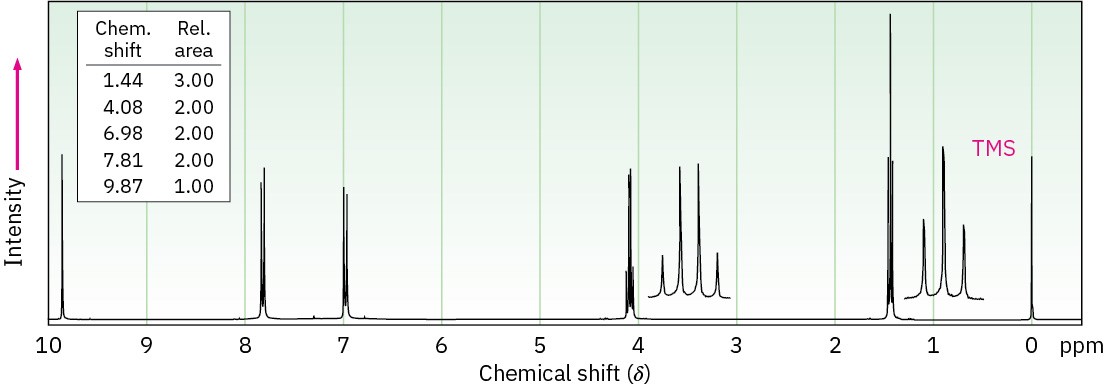
(b) C4H6O
IR: 1690 cm–1
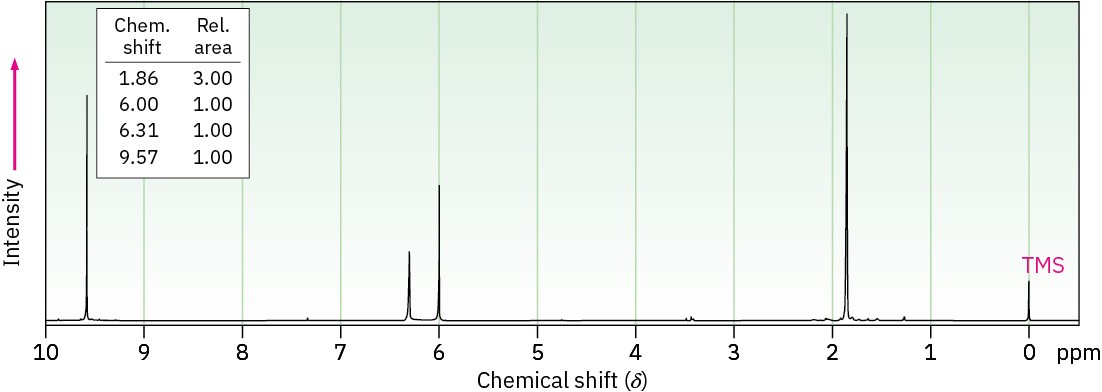
Problem 19-83
Propose structures for ketones or aldehydes that have the following 1H NMR spectra.
(a) C10H12O
IR: 1710 cm–1
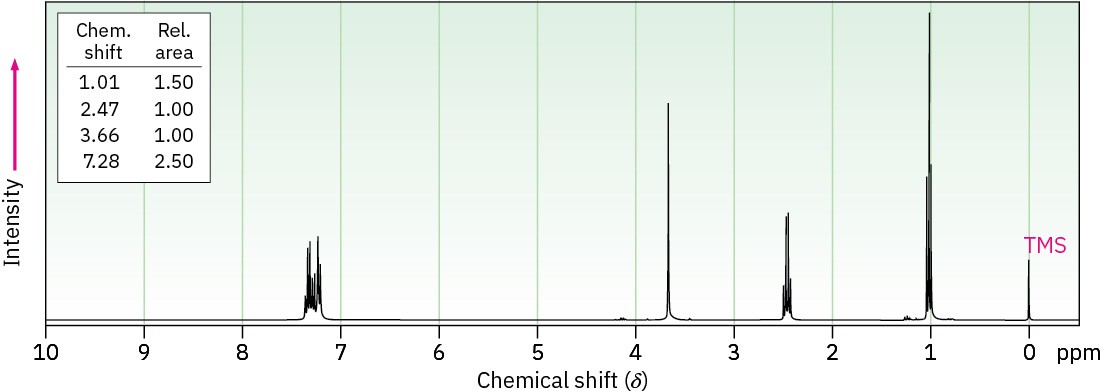
(b) C6H12O3
IR: 1715 cm–1
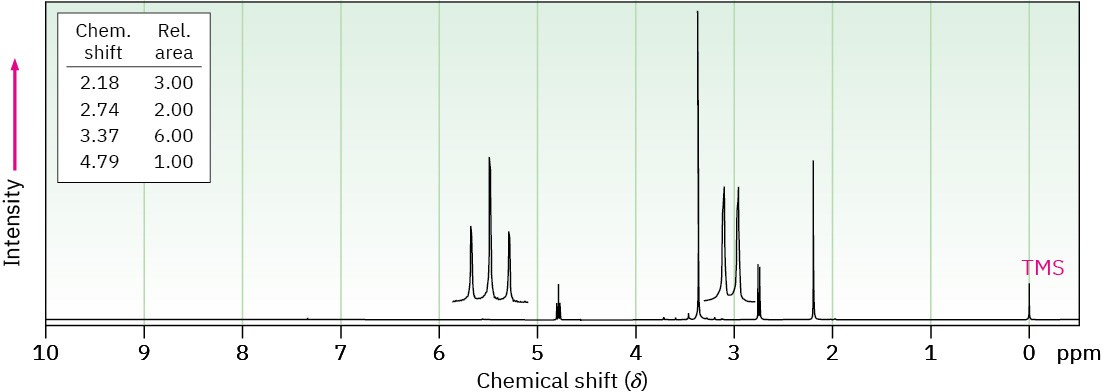
Problem 19-84
When glucose (Problem 19-51) is treated with NaBH4, reaction occurs to yield sorbitol, a polyalcohol commonly used as a food additive. Show how this reduction occurs.
Problem 19-85
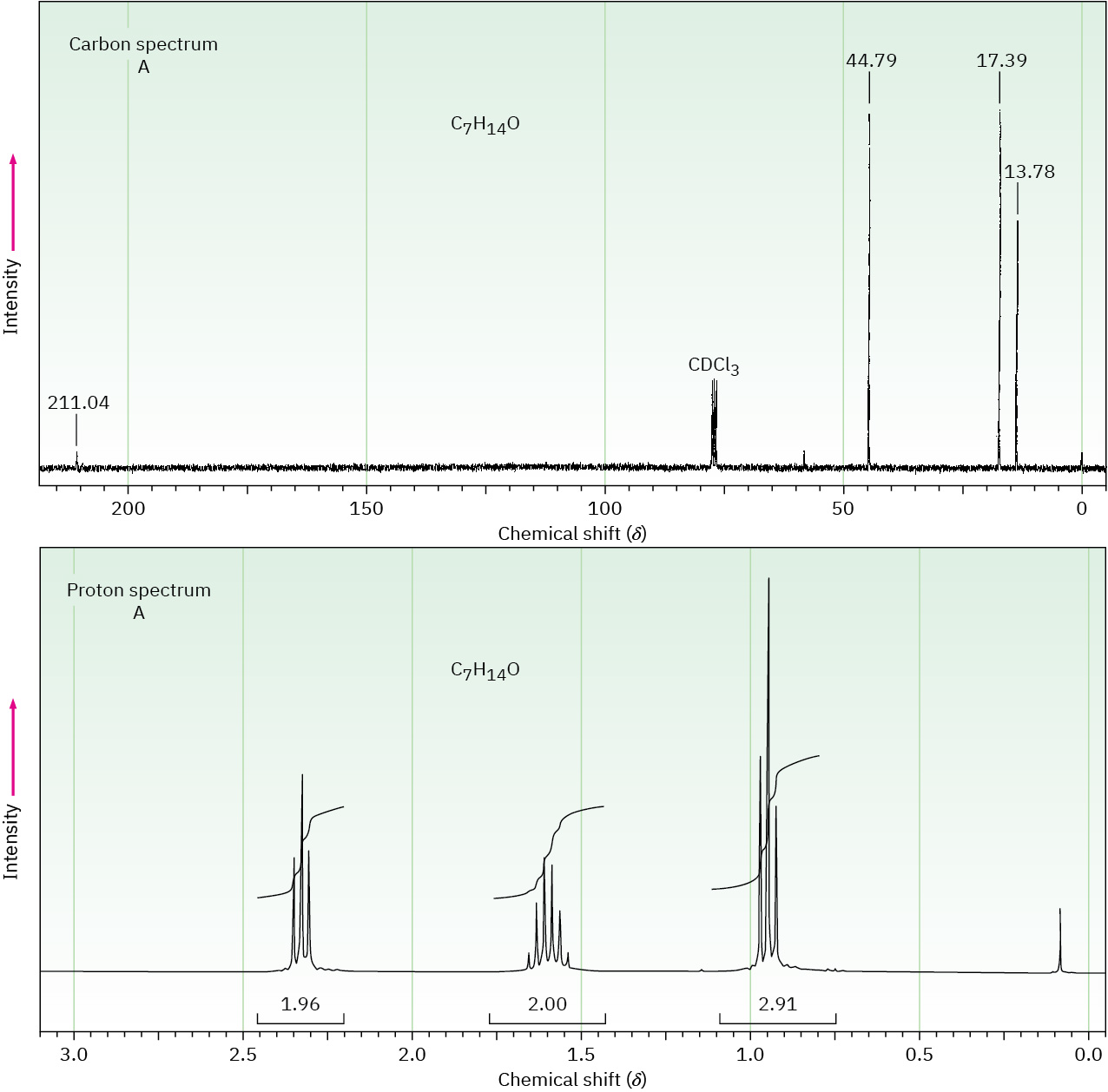
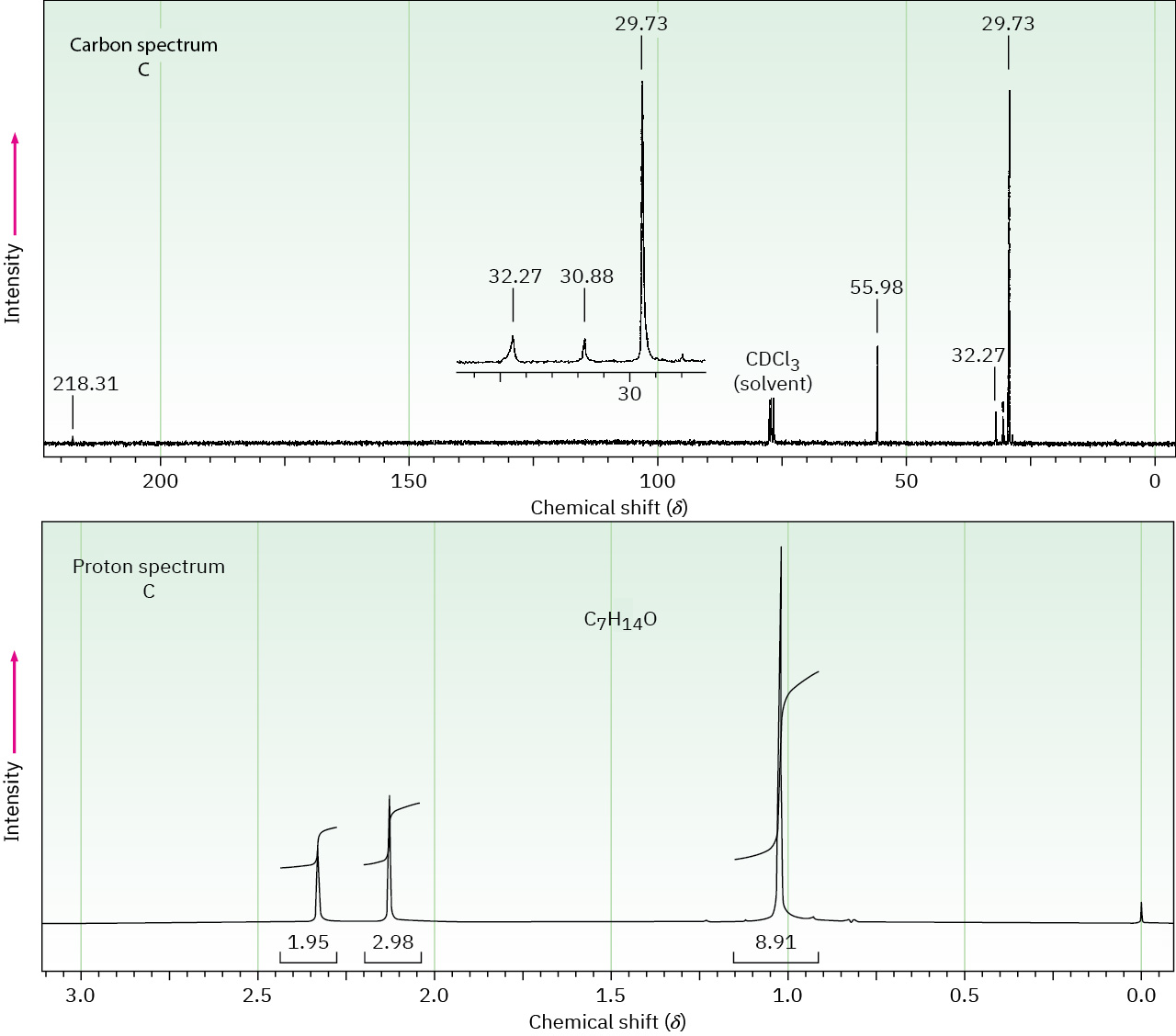
The proton and carbon NMR spectra for each of three isomeric ketones with the formula C7H14O are shown. Assign a structure to each pair of spectra.
Problem 19-86
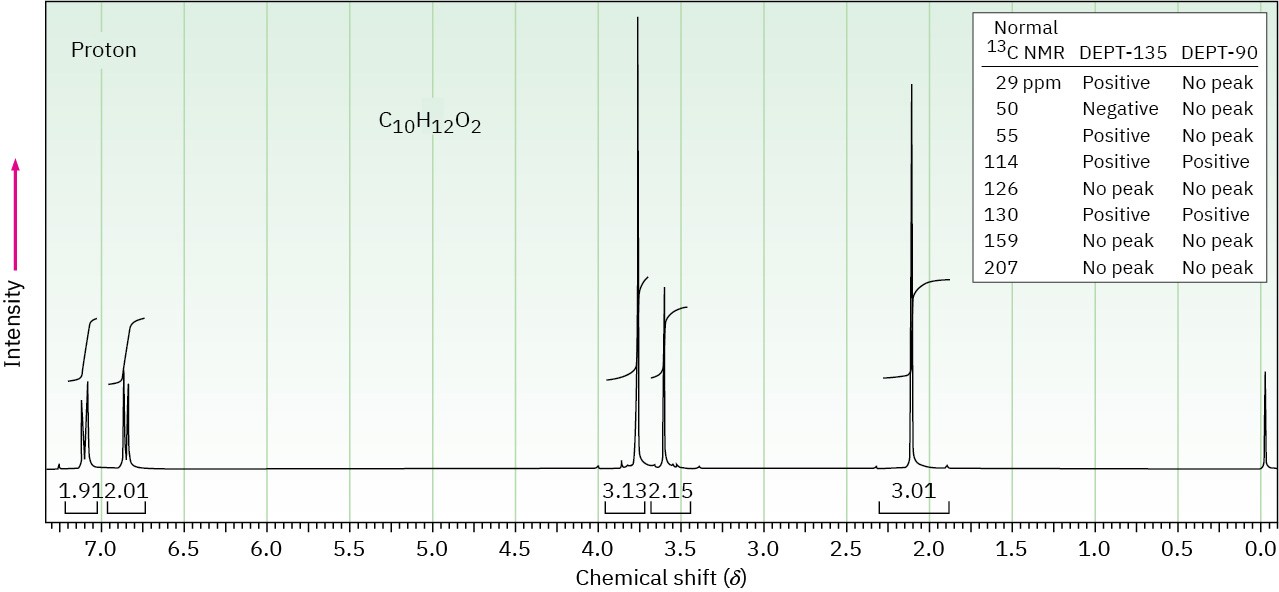
The proton NMR spectrum for a compound with formula C10H12O2 is shown below. The infrared spectrum has a strong band at 1711 cm–1. The broadband-decoupled 13C NMR spectral results are tabulated along with the DEPT-135 and DEPT-90 information. Draw the structure of this compound.