Chapter 1
Problem 1-1
(a) 1s2 2s2 2p4
(b) 1s2 2s2 2p3
(c) 1s2 2s2 2p6 3s6 3p4
Problem 1-2
(a) 2
(b) 2
(c) 6
Problem 1-3
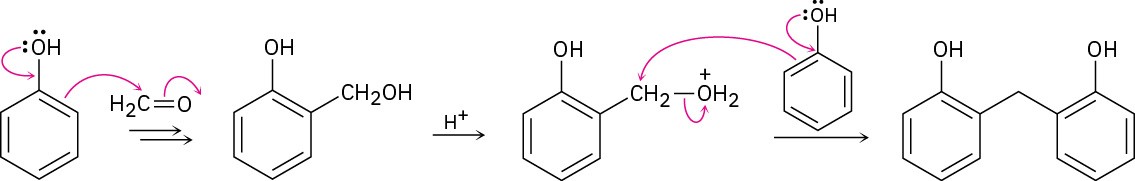
Problem 1-4
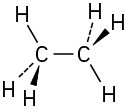
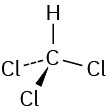
Problem 1-5
(a) CCl4
(b) AlH3
(c) CH2Cl2
(d) SiF4
(e) CH3NH2
Problem 1-6
(a)
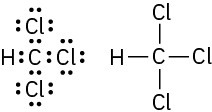
(b)
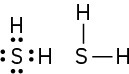
(c)
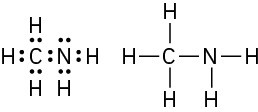
(d)
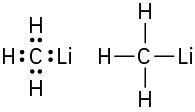
Problem 1-7
C2H7 has too many hydrogens for a compound with two carbons.
Problem 1-8
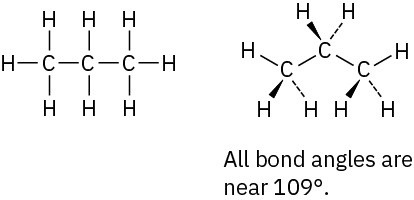
Problem 1-9
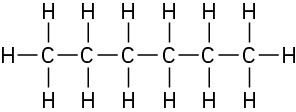
Problem 1-10
The CH3 carbon is sp3; the double-bond carbons are sp2; the C=C−C and C=C−H bond angles are approximately 120°; other bond angles are near 109°.
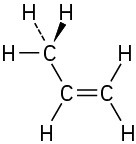
Problem 1-11
All carbons are sp2, and all bond angles are near 120°.
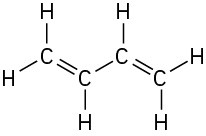
Problem 1-12
All carbons except CH3 are sp2.
Problem 1-13
The CH3 carbon is sp3; the triple-bond carbons are sp; the C≡C−C and H−C≡C bond angles are approximately 180°.
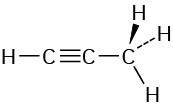
Problem 1-14
(a) O has 2 lone pairs and is sp3-hybridized.
(b) N has 1 lone pair and is sp3-hybridized.
(c) P has 1 lone pair and is sp3-hybridized.
(d) S has 2 lone pairs and is sp3-hybridized.
Problem 1-15
(a)
(b)
Problem 1-16
(a)
There are numerous possibilities, such as:
(b)
There are numerous possibilities, such as:
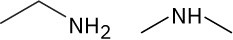
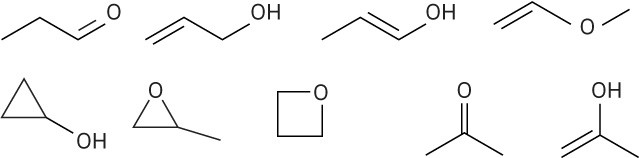
(c)
There are numerous possibilities, such as:
(d)
There are numerous possibilities, such as:
Problem 1-17
Chapter 2
Problem 2-1
(a) H
(b) Br
(c) Cl
(d) C
Problem 2-2
(a)
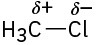
(b)
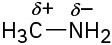
(c)
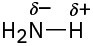
(d)
(e)
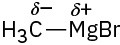
(f)
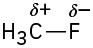
Problem 2-3
H3C−OH < H3C−MgBr < H3C−Li = H3C−F < H3C−K
Problem 2-4
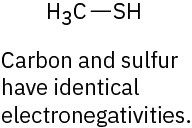
The nitrogen is electron-rich, and the carbon is electron-poor.
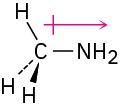
Problem 2-5
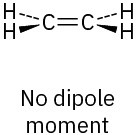
The two C–O dipoles cancel because of the symmetry of the molecule:
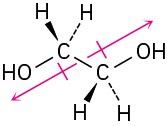
Problem 2-6
(a)
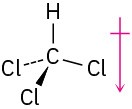
(b)
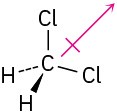
(c)
(d)
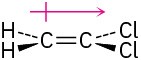
Problem 2-7
(a) For carbon: FC = 4 − 8/2 − 0 = 0 For the middle nitrogen: FC = 5 − 8/2 − 0 = +1 For the end nitrogen: FC = 5 − 4/2 − 4 = −1
(b) For nitrogen: FC = 5 − 8/2 − 0 = +1 For oxygen: FC = 6 − 2/2 − 6 = −1
(c) For nitrogen: FC = 5 − 8/2 − 0 = +1 For the triply bonded carbon: FC = 4 − 6/2 − 2 = −1
Problem 2-8
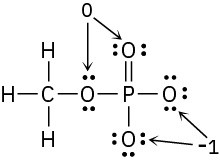
Problem 2-9
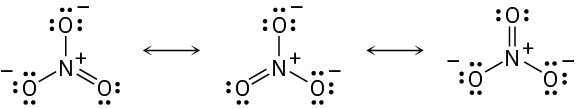
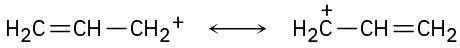
The structures in (a) are resonance forms.
Problem 2-10
(a)
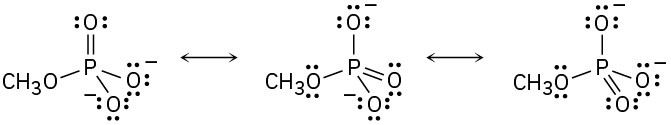
(b)
(c)
(d)
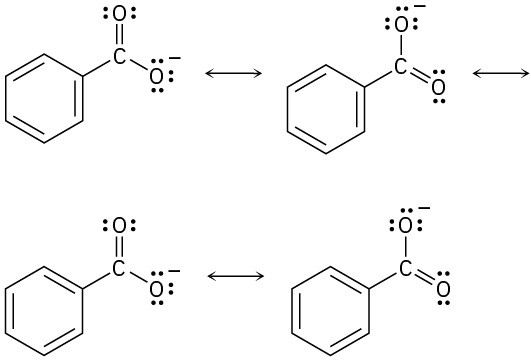
Problem 2-11
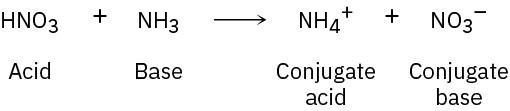
Problem 2-12
Phenylalanine is stronger.
Problem 2-13
Water is a stronger acid.
Problem 2-14
Neither reaction will take place.
Problem 2-15
Reaction will take place.
Problem 2-16
Ka = 4.9 × 10–10
Problem 2-17
(a)
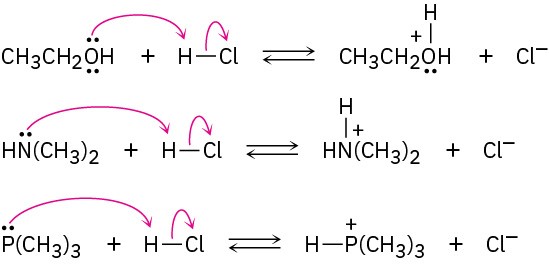
(b)
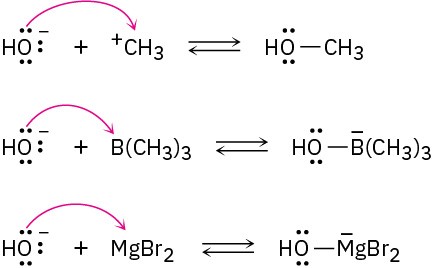
Problem 2-18
(a)
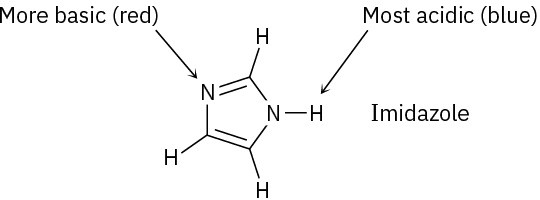
(b)
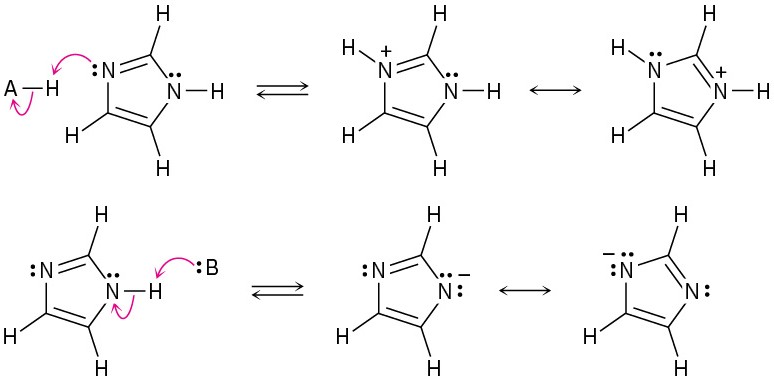
Problem 2-19
Vitamin C is water-soluble (hydrophilic); vitamin A is fat-soluble (hydrophilic).
Chapter 3
Problem 3-1
(a) Sulfide, carboxylic acid, amine
(b) Aromatic ring, carboxylic acid
(c) Ether, alcohol, aromatic ring, amide, C=C bond
Problem 3-2
(a)
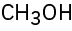
(b)
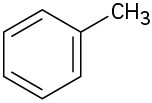
(c)
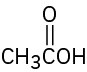
(d)
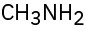
(e)
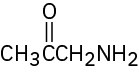
(f)
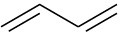
Problem 3-3
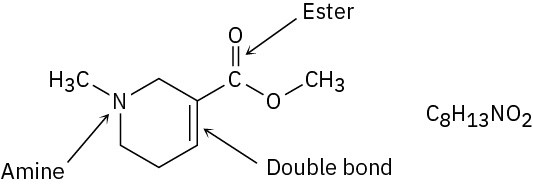
Problem 3-4
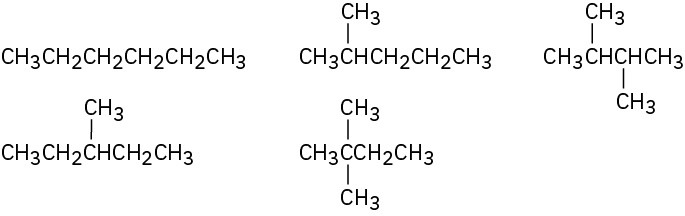
Problem 3-5
(a)
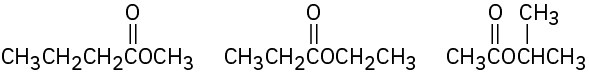
(b)
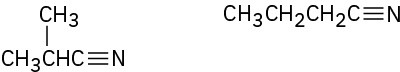
(c)

Problem 3-6
(a) Two (b) Four (c) Four
Problem 3-7
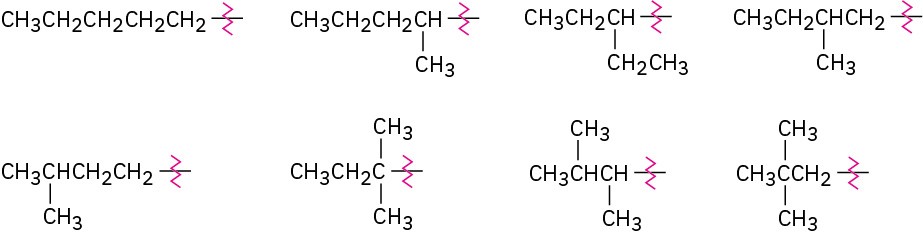
Problem 3-8
(a)
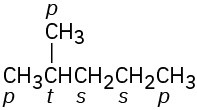
(b)
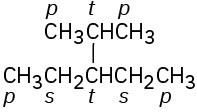
(c)
Problem 3-9
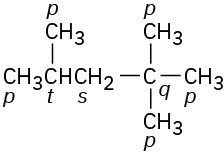
Primary carbons have primary hydrogens, secondary carbons have secondary hydrogens, and tertiary carbons have tertiary hydrogens.
Problem 3-10
(a)
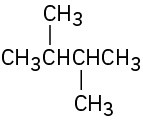
(b)
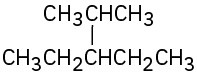
(c)
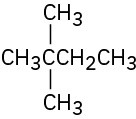
Problem 3-11
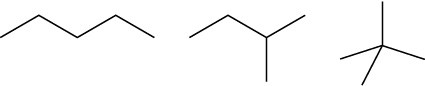
(a) Pentane, 2-methylbutane, 2,2-dimethylpropane
(b) 2,3-Dimethylpentane
(c) 2,4-Dimethylpentane
(d) 2,2,5-Trimethylhexane
Problem 3-12
(a)
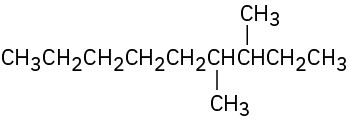
(b)
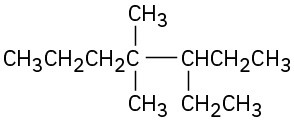
(c)
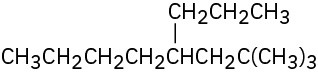
(d)
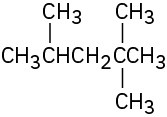
Problem 3-13
Pentyl, 1-methylbutyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl
Problem 3-14
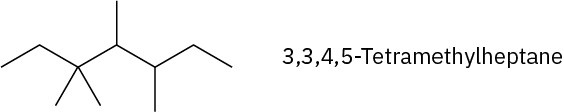
Problem 3-15
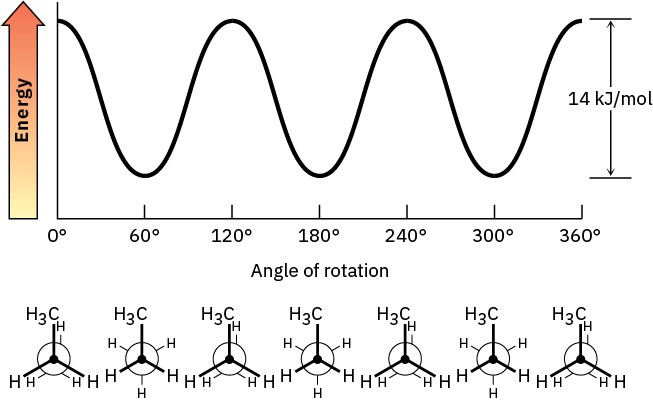
Problem 3-16
(a)
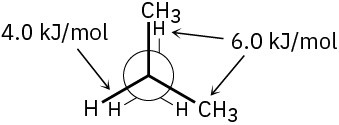
(b)
(c)
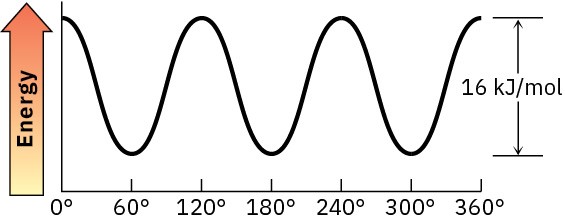
(d)
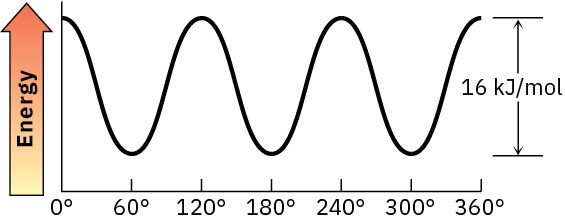
Problem 3-17
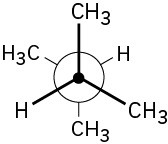
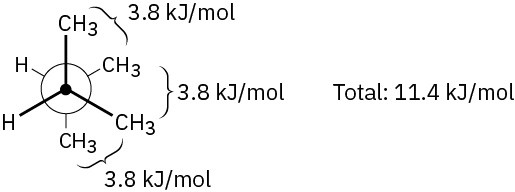
Problem 3-18
Chapter 4
Problem 4-1
(a) 1,4-Dimethylcyclohexane
(b) 1-Methyl-3-propylcyclopentane
(c) 3-Cyclobutylpentane
(d) 1-Bromo-4-ethylcyclodecane
(e) 1-Isopropyl-2-methylcyclohexane
(f) 4-Bromo-1-tert-butyl-2-methylcycloheptane
Problem 4-2
(a)
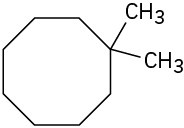
(b)
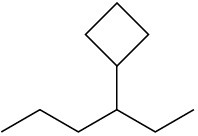
(c)
(d)
Problem 4-3
3-Ethyl-1,1-dimethylcyclopentane
Problem 4-4
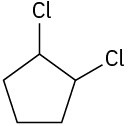
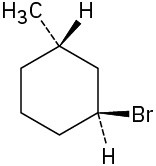
(a) trans-1-Chloro-4-methylcyclohexane (b) cis-1-Ethyl-3-methylcycloheptane
Problem 4-5
(a)
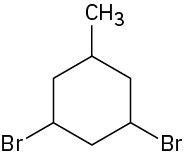
(b)
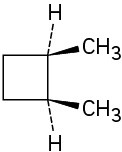
(c)
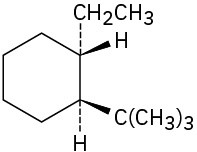
Problem 4-6
The two hydroxyl groups are cis. The two side chains are trans.
Problem 4-7
(a) cis-1,2-Dimethylcyclopentane
(b) cis-1-Bromo-3-methylcyclobutane
Problem 4-8
Six interactions; 21% of strain
Problem 4-9
The cis isomer is less stable because the methyl groups nearly eclipse each other.
Problem 4-10
Ten eclipsing interactions; 40 kJ/mol; 35% is relieved.
Problem 4-11
Conformation (a) is more stable because the methyl groups are farther apart.
Problem 4-12
Problem 4-13
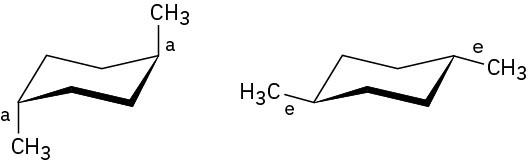
Problem 4-14
Before the ring-flip, red and blue are equatorial and green is axial. After the ring-flip, red and blue are axial and green is equatorial.
Problem 4-15
4.2 kJ/mol
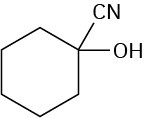
Problem 4-16
Cyano group points straight up.
Problem 4-17
Equatorial = 70%; axial = 30%
Problem 4-18
(a) 2.0 kJ/mol (axial Cl)
(b)11.4 kJ/mol (axial CH3)
(c) 2.0 kJ/mol (axial Br)
(d) 8.0 kJ/mol (axial CH2CH3)
Problem 4-19
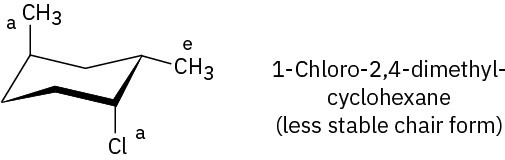
Problem 4-20
trans-Decalin is more stable because it has no 1,3-diaxial interactions.
Problem 4-21
Both ring-fusions are trans.
Chapter 5
Problem 5-1 Chiral: screw, shoe Problem 5-2
(a)
(b)
(c)
Problem 5-3

Problem 5-4 (a)
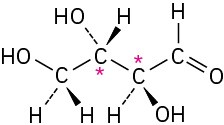
(b)
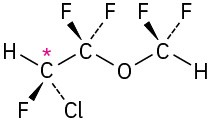
Problem 5-5 Levorotatory Problem 5-6
+16.1°
Problem 5-7 (a)
−Br (b)
−Br (c)
−CH2CH3
(d)
−OH
(e)
−CH2OH
(f)
−CH=O
Problem 5-8 (a)
−OH, −CH2CH2OH, −CH2CH3, −H
(b)
−OH, −CO2CH3, −CO2H, −CH2OH
(c)
−NH2, −CN, −CH2NHCH3, −CH2NH2
(d)
−SSCH3, −SH, −CH2SCH3, −CH3
Problem 5-9 (a)
S
(b)
R
(c)
S
Problem 5-10 (a)
S
(b)
S
(c)
R
Problem 5-11
Problem 5-12
S
Problem 5-13
Compound (a) is D-erythrose 4-phosphate, (d) is its enantiomer, and (b) and (c) are diastereomers.
Problem 5-14
Five chirality centers and 25 = 32 stereoisomers Problem 5-15
S,S
Problem 5-16
Compounds (a) and (d) are meso. Problem 5-17
Compounds (a) and (c) have meso forms.
Problem 5-18
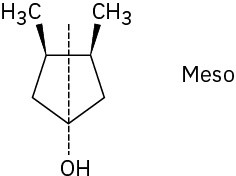
Problem 5-20
Two diastereomeric salts: (R)-lactic acid plus (S)-1-phenylethylamine and (S)-lactic acid plus (S)-1-phenylethylamine
Problem 5-21 (a)
Constitutional isomers (b)
Diastereomers Problem 5-22 (a)
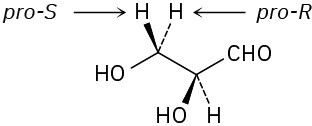
(b)
Problem 5-23 (a)
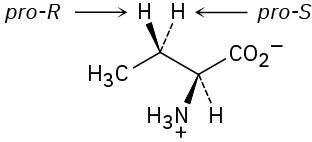
(b)
Problem 5-24 (S)-Lactate Problem 5-25
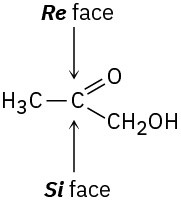
The −OH adds to the Re face of C2, and −H adds to the Re face of C3. The overall addition has anti stereochemistry.
Chapter 6
Problem 6-1
(a) Substitution (b) Elimination (c) Addition
Problem 6-2
(a) Carbon is electrophilic.
(b) Sulfur is nucleophilic.
(c) Nitrogens are nucleophilic.
(d) Oxygen is nucleophilic; carbon is electrophilic.
Problem 6-3
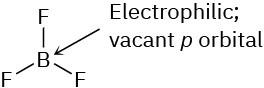
Problem 6-4
Bromocyclohexane; chlorocyclohexane
Problem 6-5
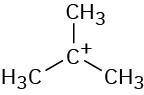
The mechanism is shown in Figure 6.4
Problem 6-6
(a)
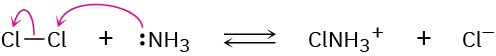
(b)
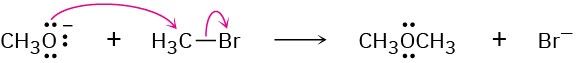
(c)
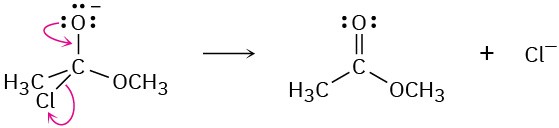
Problem 6-7
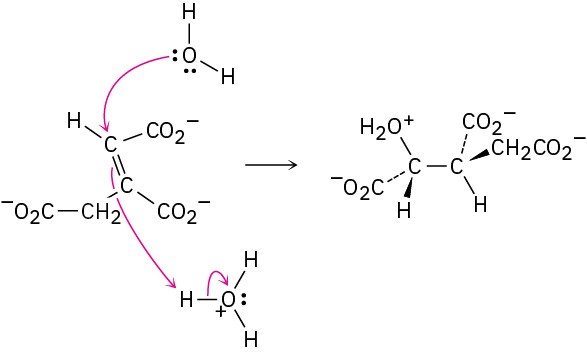
Problem 6-8
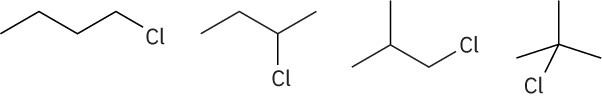
1-Chloro-2-methylpentane, 2-chloro-2-methylpentane, 3-chloro-2-methylpentane, 2- chloro-4-methylpentane, 1-chloro- 4-methylpentane
Problem 6-9
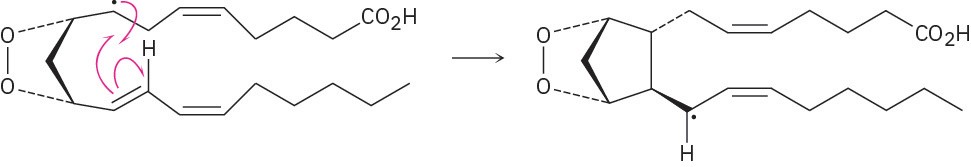
Problem 6-10
Negative ΔG° is favored.
Problem 6-11
Larger Keq is more exergonic.
Problem 6-12
Lower ΔG‡ is faster.
Problem 6-13
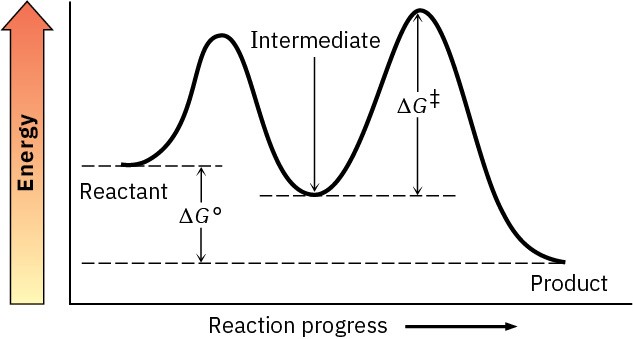
Chapter 7
Problem 7-1
(a)1
(b) 2
(c) 2
Problem 7-2
(a)5
(b) 5
(c) 3
(d) 1
(e) 6
(f) 5
Problem 7-3
C16H13ClN2O
Problem 7-4
(a) 3,4,4-Trimethyl-1-pentene
(b)3-Methyl-3-hexene
(c) 4,7-Dimethyl-2,5-octadiene
(d) 6-Ethyl-7-methyl-4-nonene
Problem 7-5
(a)
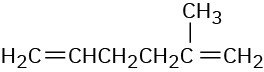
(b)
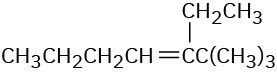
(c)
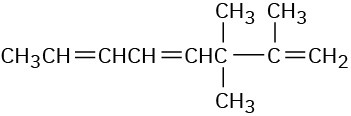
(d)
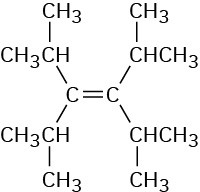
Problem 7-6
(a) 1,2-Dimethylcyclohexene
(b) 4,4-Dimethylcycloheptene
(c) 3-Isopropylcyclopentene
Problem 7-7
(a) 2,5,5-Trimethylhex-2-ene
(b) 2,3-Dimethylcyclohexa-1,3-diene
Problem 7-8
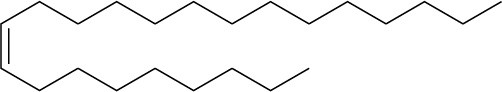
Problem 7-9
Compounds (c), (e), and (f) have cis–trans isomers.
Problem 7-10
(a) cis-4,5-Dimethyl-2-hexene
(b) trans-6-Methyl-3-heptene
Problem 7-11
(a)−CH3
(b)−Cl
(c) −CH=CH2
(d) −OCH3
(e) −CH=O
(f) −CH=O
Problem 7-12
(a) −Cl, −OH, −CH3, −H
(b) −CH2OH, −CH=CH2, −CH2CH3, −CH3
(c) −CO2H, −CH2OH, −C≡N, −CH2NH2
(d) −CH2OCH3, −C≡N, −C≡CH, −CH2CH3
Problem 7-13
(a) Z
(b)E
(c)Z
(d)E
Problem 7-14
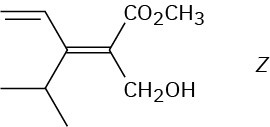
Problem 7-15
(a)2-Methylpropene is more stable than 1-butene.
(b)trans-2-Hexene is more stable than cis-2-hexene.
(c) Methylcyclohexene is more stable than 3-methylcyclohexene.
Problem 7-16
(a) Chlorocyclohexane
(b) Bromo-2-methylpentane
(c) 4-Methyl-2-pentanol
(d) 1-Bromo-1-methylcyclohexane
Problem 7-17
(a) Cyclopentene
(b) 1-Ethylcyclohexene or ethylidenecyclohexane
(c) 3-Hexene
(d) Vinylcyclohexane (cyclohexylethylene)
Problem 7-18
(a)
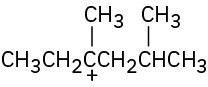
(b)
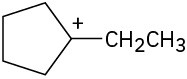
Problem 7-19
In the conformation shown, only the methyl- group C−H that is parallel to the carbocation p orbital can show hyperconjugation.
Problem 7-20
The second step is exergonic; the transition state resembles the carbocation.
Problem 7-21
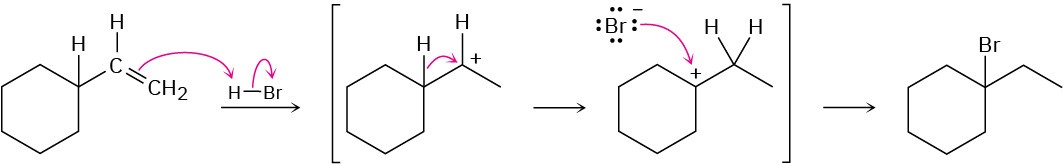
Chapter 8
Problem 8-1
2-Methyl-2-butene and 2-methyl-1-butene
Problem 8-2
Five
Problem 8-3
trans-1,2-Dichloro-1,2-dimethylcyclohexane
Problem 8-4
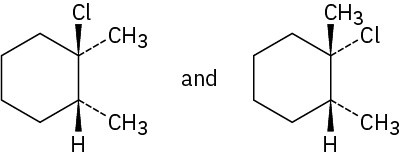
Problem 8-5
trans-2-Bromocyclopentanol
Problem 8-6
Markovnikov
Problem 8-7
(a) 2-Pentanol (b) Methyl-2-pentanol
Problem 8-8
(a) Oxymercuration of 2-methyl-1-hexene or 2-methyl-2-hexene
(b) Oxymercuration of cyclohexylethylene or hydroboration of ethylidenecyclohexane
Problem 8-9
(a)
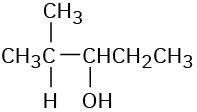
(b)
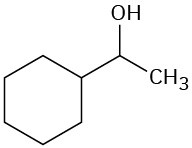
Problem 8-10
(a) Methyl-1-butene (b) 2-Methyl-2-butene (c) Methylenecyclohexane
Problem 8-11
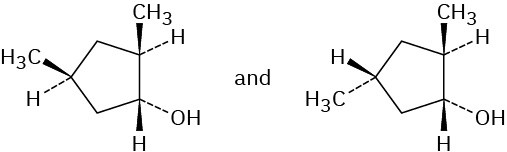
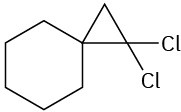
Problem 8-12 (a) 2-Methylpentane (b) 1,1-Dimethylcyclopentane (c) tert-Butylcyclohexane
Problem 8-13

Problem 8-14
(a) Methylcyclohexene
(b) Methyl-2-pentene
(c) 1,3-Butadiene
Problem 8-15
(a) CH3COCH2CH2CH2CH2CO2H
(b) CH3COCH2CH2CH2CH2CHO
Problem 8-16
(a) Methylpropene
(b) Hexene
Problem 8-17
(a)
(b)
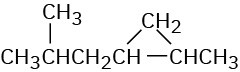
Problem 8-18
(a) H2C═CHOCH3
(b) ClCH═CHCl
Problem 8-19

Problem 8-20
An optically inactive, non-50 : 50 mixture of two racemic pairs: (2R,4R) + (2S,4S) and (2R,4S) + (2S,4R)
Problem 8-21
Non-50 : 50 mixture of two racemic pairs: (1S,3R) + (1R,3S) and (1S,3S) + (1R,3R)
Chapter 9
Problem 9-1
(a) 2,5-Dimethyl-3-hexyne
(b) 3,3-Dimethyl-1-butyne (
c) 3,3-Dimethyl-4-octyne
(d) 2,5,5-Trimethyl-3-heptyne
(e) 2,4-Octadiene-6-yne
Problem 9-2
1-Hexyne, 2-hexyne, 3-hexyne, 3-methyl-1-pentyne, 4-methyl-1-pentyne, 4-methyl-2- pentyne, 3,3-dimethyl-1-butyne
Problem 9-3
(a) 1,1,2,2-Tetrachloropentane
(b) Bromo-1-cyclopentylethylene
(c) Bromo-2-heptene and 3-bromo-2-heptene
Problem 9-4
(a) 4-Octanone
(b) 2-Methyl-4-octanone and 7-methyl-4-octanone
Problem 9-5
(a) Pentyne
(b) Pentyne
Problem 9-6
(a) C6H5C≡CH
(b) 2,5-Dimethyl-3-hexyne
Problem 9-7
(a) Mercuric sulfate–catalyzed hydration of phenylacetylene
(b) Hydroboration/oxidation of cyclopentylacetylene
Problem 9-8
(a) Reduce 2-octyne with Li/NH3.
(b) Reduce 3-heptyne with H2/Lindlar catalyst.
(c) Reduce 3-methyl-1-pentyne.
Problem 9-9
No: (a), (c), (d); yes: (b)
Problem 9-10
(a) 1-Pentyne + CH3I, or propyne + CH3CH2CH2I
(b) 3-Methyl-1-butyne + CH3CH2I
(c) Cyclohexylacetylene + CH3I Problem 9-11

Problem 9-12
(a) KMnO4, H3O+
(b) H2/Lindlar
(c) 1. H2/Lindlar; 2. HBr
(d) 1. H2/Lindlar; 2. BH3; 3. NaOH, H2O2
(e) 1. H2/Lindlar; 2. Cl2
(f) O3
Problem 9-13
(a) 1. HC≡CH + NaNH2 ; 2. CH3(CH2)6CH2Br; 3. 2 H2/Pd
(b) HC≡CH + NaNH2 ; 2. (CH3)3CCH2CH2I; 3. 2 H2/Pd
(c) 1. HC≡CH + NaNH2 ; 2. CH3CH2CH2CH2I; 3. BH3; 4. H2O2
(d) 1. HC≡CH + NaNH2 ; 2. CH3CH2CH2CH2CH2I; 3. HgSO4, H3O+
Chapter 10
Problem 10-1
(a) 1-Iodobutane
(b) 1-Chloro-3-methylbutane
(c) 1,5-Dibromo-2,2-dimethylpentane
(d) 1,3-Dichloro-3-methylbutane
(e) Chloro-3-ethyl-4-iodopentane
(f) Bromo-5-chlorohexane
Problem 10-2
(a)
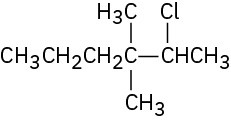
(b)
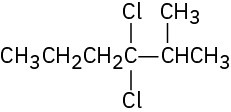
(c)
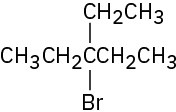
(d)
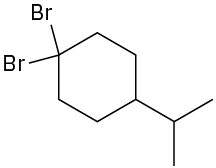
(e)
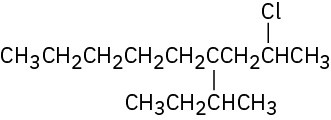
(f)
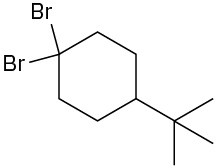
Problem 10-3
Chiral: 1-chloro-2-methylpentane, 3-chloro-2-methylpentane, 2-chloro-4-methylpentane Achiral: 2-chloro-2-methylpentane, 1-chloro-4-methylpentane
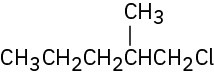
Problem 10-4
1-Chloro-2-methylbutane (29%), 1-chloro-3-methylbutane (14%), 2-chloro-2-
methylbutane (24%), 2-chloro-3-methylbutane (33%)
Problem 10-5
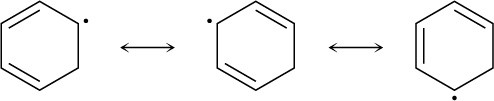
Problem 10-6
The intermediate allylic radical reacts at the more accessible site and gives the more highly substituted double bond.
Problem 10-7
(a) 3-Bromo-5-methylcycloheptene and 3-bromo-6-methylcycloheptene
(b)Four products
Problem 10-8
(a) 2-Methyl-2-propanol + HCl
(b) 4-Methyl-2-pentanol + PBr3
(c) 5-Methyl-1-pentanol + PBr3
(d) 3,3-Dimethyl-cyclopentanol + HF, pyridine
Problem 10-9
Both reactions occur.
Problem 10-10
React Grignard reagent with D2O.
Problem 10-11
(a) 1. NBS; 2. (CH3)2CuLi
(b) 1. Li; 2. CuI; 3. CH3CH2CH2CH2Br
(c) 1. BH3; 2. H2O2, NaOH; 3. PBr3; 4. Li, then CuI; 5. CH3(CH2)4Br
Problem 10-12
(a)
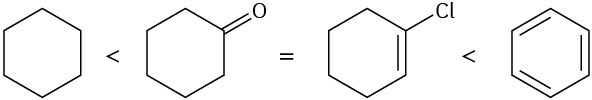
(b)
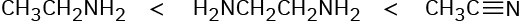
Problem 10-13
(a) Reduction (b) Neither
Chapter 11
Problem 11-1
(R)-1-Methylpentyl acetate, CH3CO2CH(CH3)CH2CH2CH2CH3
Problem 11-2
(S)-2-Butanol
Problem 11-3

Problem 11-4
(a) 1-Iodobutane
(b) 1-Butanol
(c) 1-Hexyne
(d) Butylammonium bromide
Problem 11-5
(a) (CH3)2N−
(b) (CH3)3N
(c) H2S
Problem 11-6
CH3OTos > CH3Br > (CH3)2CHCl > (CH3)3CCl
Problem 11-7
Similar to protic solvents
Problem 11-8
Racemic 1-ethyl-1-methylhexyl acetate
Problem 11-9
90.1% racemization, 9.9% inversion
Problem 11-10
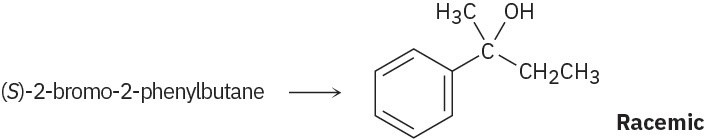
Problem 11-11
H2C═CHCH(Br)CH3 > CH3CH(Br)CH3 > CH3CH2Br > H2C═CHBr
Problem 11-12
The same allylic carbocation intermediate is formed.
Problem 11-13
(a) SN1
(b) SN2
Problem 11-14
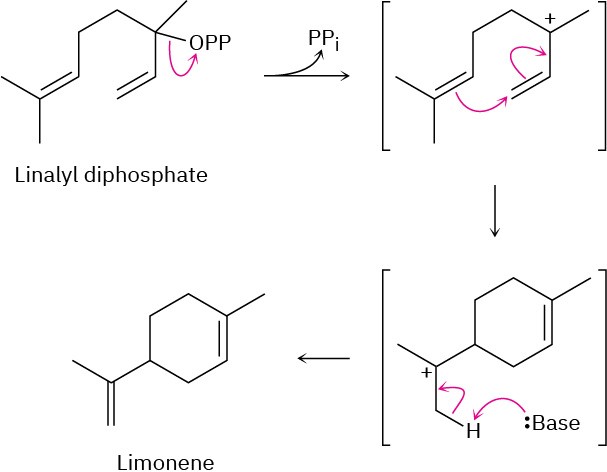
Problem 11-15
(a) Major: 2-methyl-2-pentene; minor: 4-methyl-2-pentene
(b) Major: 2,3,5-trimethyl-2-hexene; minor: 2,3,5-trimethyl-3-hexene and 2-isopropyl-4- methyl-1-pentene
(c) Major: ethylidenecyclohexane; minor: cyclohexylethylene
Problem 11-16
(a) 1-Bromo-3,6-dimethylheptane (b) 4-Bromo-1,2-dimethylcyclopentane
Problem 11-17
(Z)-1-Bromo-1,2-diphenylethylene
Problem 11-18
(Z)-3-Methyl-2-pentene
Problem 11-19
Cis isomer reacts faster because the bromine is axial.
Problem 11-20
(a) SN2 (b) E2 (c) SN1 (d) E1cB
Chapter 12
Problem 12-1
C19H28O2
Problem 12-2
(a) 2-Methyl-2-pentene (b) 2-Hexene
Problem 12-3
(a) 43, 71
(b) 82
(c) 58
(d) 86
Problem 12-4
102 (M+), 84 (dehydration), 87 (alpha cleavage), 59 (alpha cleavage)
Problem 12-5
X-ray energy is higher; λ = 9.0 × 10−6 m is higher in energy.
Problem 12-6
(a) 2.4 × 106 kJ/mol] (b) 4.0 × 104 kJ/mol (c) 2.4 × 103 kJ/mol (d) 2.8 × 102 kJ/mol (e) 6.0 kJ/mol (f) 4.0 × 10−2 kJ/mol
Problem 12-7
(a) Ketone or aldehyde (b) Nitro compound (c) Carboxylic acid
Problem 12-8
(a) CH3CH2OH has an −OH absorption. (b) 1-Hexene has a double-bond absorption. (c) CH3CH2CO2H has a very broad −OH absorption.
Problem 12-9
1450–1600 cm−1: aromatic ring; 2100 cm−1: C≡C; 3300 cm−1: C≡C−H
Problem 12-10
(a) 1715, 1640, 1250 cm−1
(b) 1730, 2100, 3300 cm−1
(c) 1720, 2500–3100, 3400–3650 cm−1
Problem 12-11
1690, 1650, 2230 cm−1
Chapter 13
Problem 13-1
7.5 × 10−5 kJ/mol for 19F; 8.0 × 10−5 kJ/mol for 1H
Problem 13-2
1.2 × 10−4 kJ/mol
Problem 13-3
The vinylic C − H protons are nonequivalent.
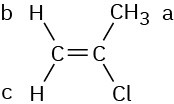
Problem 13-4
(a) 7.27 δ
(b) 3.05 δ
(c) 3.46 δ
(d) 5.30 δ
Problem 13-5 (a) 420 Hz (b) 2.1 δ (c) 1050 Hz
Problem 13-6
(a) 1.43 δ (b) 2.17 δ (c) 7.37 δ (d) 5.30 δ (e) 9.70 δ (f) 2.12 δ
Problem 13-7
There are seven kinds of protons labeled. The types and expected range of absorption of each follow. a: ether, 3.5–4.5 δ; b: aryl, 6.5–8.0 δ; c: aryl, 6.5–8.0; d: vinylic, 4.5–6.5 δ; e: vinylic, 4.5–6.5 δ; f: alkyl (secondary), 1.2–1.6 δ; g: alkyl (primary), 0.7–1.3 δ.
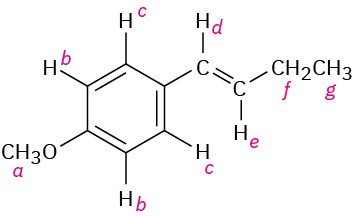
Problem 13-8
Two peaks; 3 : 2 ratio
Problem 13-9
(a) −CHBr2, quartet; −CH3, doublet (b) CH3O−, singlet; −OCH2 −, triplet; −CH2Br, triplet (c) ClCH2− , triplet; −CH2−, quintet (d) CH3− , triplet; −CH2− , quartet; −CH− , septet; (CH3)2, doublet (e) CH3−, triplet; −CH2−, quartet; −CH−, septet; (CH3)2, doublet (f) =CH, triplet, −CH2−, doublet, aromatic C−H, two multiplets
Problem 13-10
(a) CH3OCH3
(b) CH3CH(Cl)CH3
(c) ClCH2CH2OCH2CH2Cl
(d) CH3CH2CO2CH3 or CH3CO2CH2CH3
Problem 13-11
CH3CH2OCH2CH3
Problem 13-12 (a) Enantiotopic (b) Diastereotopic (c) Diastereotopic (d) Diastereotopic (e) Diastereotopic (f) Homotopic
Problem 13-13
(a) 2
(b) 4
(c) 3
(d) 4
(e) 5
(f) 3
Problem 13-14
4
Problem 13-15
J1–2 = 16 Hz; J2–3 = 8 Hz
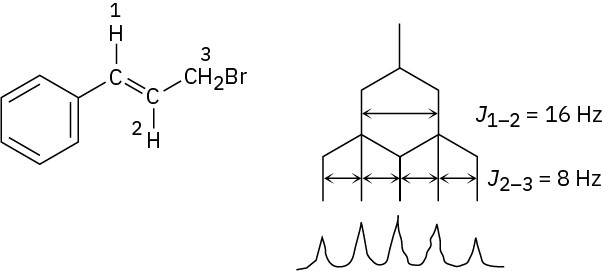
Problem 13-16
Chloro-1-methylcyclohexane has a singlet methyl absorption.
Problem 13-17
(a) 4
(b) 7
(c) 4
(d) 5
(e) 5
(f) 7
Problem 13-18
(a) 1,3-Dimethylcyclopentene (b) Methylpentane (c) 1-Chloro-2-methylpropane
Problem 13-19
−CH3, 9.3 δ; −CH2− , 27.6 δ; C=O, 174.6 δ;− OCH3, 51.4 δ
Problem 13-20
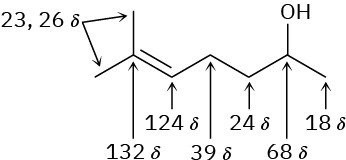
Problem 13-21
Problem 13-22
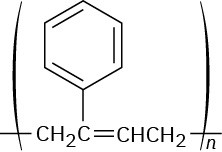
Problem 13-23
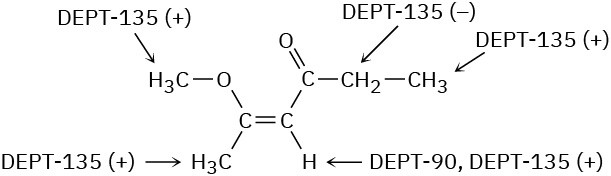
A DEPT-90 spectrum would show two absorptions for the non-Markovnikov product (RCH=CHBr) but no absorptions for the Markovnikov product (RBrC=CH2).
Chapter 14
Problem 14-1
Expected ΔH°hydrog for allene is −252 kJ/mol. Allene is less stable than a nonconjugated diene, which is less stable than a conjugated diene.
Problem 14-2
1-Chloro-2-pentene, 3-chloro-1-pentene, 4-chloro-2-pentene
Problem 14-3
4-Chloro-2-pentene predominates in both.
Problem 14-4
1,2 Addition: 6-bromo-1,6-dimethylcyclohexene 1,4 Addition: 3-bromo-1,2- dimethylcyclohexene
Problem 14-5
Interconversion occurs by SN1 dissociation to a common intermediate cation.
Problem 14-6
The double bond is more highly substituted.
Problem 14-7
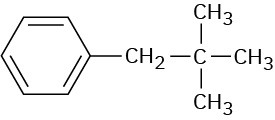
Problem 14-8
Good dienophiles: (a), (d)
Problem 14-9
Compound (a) is s-cis. Compound (c) can rotate to s-cis.
Problem 14-10
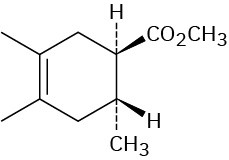
Problem 14-11
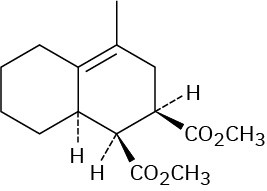
Problem 14-12
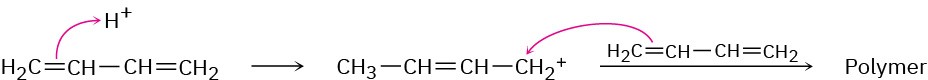
Problem 14-13
300–600 kJ/mol; UV energy is greater than IR or NMR energy.
Problem 14-14
1.46 × 10−5 M
Problem 14-15
All except (a) have UV absorptions.
Chapter 15
Problem 15-1
(a) Meta (b) Para (c) Ortho
Problem 15-2
(a) m-Bromochlorobenzene (b) (3-Methylbutyl)benzene (c) p-Bromoaniline (d) 2,5-Dichlorotoluene (e) 1-Ethyl-2,4-dinitrobenzene (f) 1,2,3,5-Tetramethylbenzene
Problem 15-3
(a)
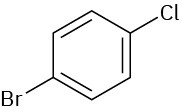
(b)
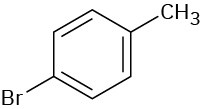
(c)
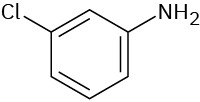
(d)
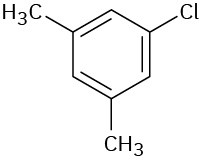
Problem 15-4
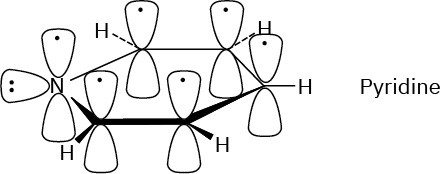
Pyridine has an aromatic sextet of electrons.
Problem 15-5
Cyclodecapentaene is not flat because of steric interactions.
Problem 15-6
All C–C bonds are equivalent; one resonance line in both 1H and 13C NMR spectra.
Problem 15-7
The cyclooctatetraenyl dianion is aromatic (ten π electrons) and flat.
Problem 15-8
Problem 15-9
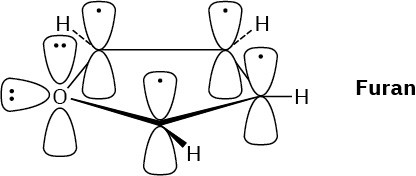
Problem 15-10
The thiazolium ring has six π electrons.
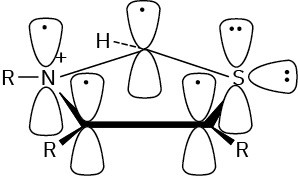
Problem 15-11 Yes, it’s aromatic.
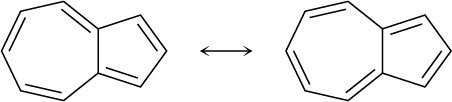
Problem 15-12
The three nitrogens in double bonds each contribute one; the remaining nitrogen contributes two.
Chapter 16
Problem 16-1
o-, m-, and p-Bromotoluene
Problem 16-2
Problem 16-3
o-xylene: 2; p-xylene: 1; m-xylene: 3
Problem 16-4
D+ does electrophilic substitutions on the ring.
Problem 16-5
No rearrangement: (a), (b), (e)
Problem 16-6
tert-Butylbenzene
Problem 16-7
(a) (CH3)2CHCOCl
(b) PhCOCl
Problem 16-8
(a)Phenol > Toluene > Benzene > Nitrobenzene
(b)Phenol > Benzene > Chlorobenzene > Benzoic acid
(c) Aniline > Benzene > Bromobenzene > Benzaldehyde
Problem 16-9
(a) o– and p-Bromonitrobenzene (b) m-Bromonitrobenzene (c) o– and p-Chlorophenol (d) o– and p-Bromoaniline
Problem 16-10
Alkylbenzenes are more reactive than benzene itself, but acylbenzenes are less reactive.
Problem 16-11
Toluene is more reactive; the trifluoromethyl group is electron-withdrawing.
Problem 16-12
The nitrogen electrons are donated to the nearby carbonyl group by resonance and are less available to the ring.
Problem 16-13
The meta intermediate is most favored.
Problem 16-14
(a) Ortho and para to −OCH3 (b) Ortho and para to −NH2 (c) Ortho and para to −Cl
Problem 16-15
(a) Reaction occurs ortho and para to the −CH3 group.
(b) Reaction occurs ortho and para to the −OCH3 group.
Problem 16-16
The phenol is deprotonated by KOH to give an anion that carries out a nucleophilic acyl substitution reaction on the fluoronitrobenzene.
Problem 16-17
Only one benzyne intermediate can form from p-bromotoluene; two different benzyne intermediates can form from m-bromotoluene.
Problem 16-18
(a) m-Nitrobenzoic acid (b) p–tert-Butylbenzoic acid
Problem 16-19
A benzyl radical is more stable than a primary alkyl radical by 52 kJ/mol and is similar in stability to an allyl radical.
Problem 16-20
1. CH3CH2Cl, AlCl3; 2. NBS; 3. KOH, ethanol
Problem 16-21
1. PhCOCl, AlCl3; 2. H2/Pd
Problem 16-22
(a) 1. HNO3, H2SO4; 2. Cl2, FeCl3
(b) 1. CH3COCl, AlCl3; 2. Cl2, FeCl3; 3. H2/Pd
(c) 1. CH3CH2COCl, AlCl3; 2. Cl2, FeCl3; 3. H2/Pd; 4. HNO3, H2SO4
(d) 1. CH3Cl, AlCl3; 2. Br2, FeBr3; 3. SO3, H2SO4
Problem 16-23
(a) Friedel–Crafts acylation does not occur on a deactivated ring.
(b) Rearrangement occurs during Friedel–Crafts alkylation with primary halides; chlorination occurs ortho to the alkyl group.
Chapter 17
Problem 17-1
(a) 5-Methyl-2,4-hexanediol (b) 2-Methyl-4-phenyl-2-butanol (c) 4,4-Dimethylcyclohexanol (d) trans-2-Bromocyclopentanol (e) 4-Bromo-3-methylphenol (f) 2-Cyclopenten-1-ol
Problem 17-2
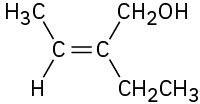
(a)
(b)
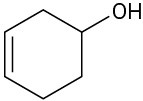
(c)
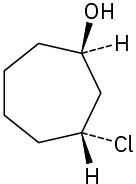
(d)
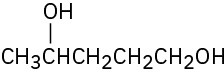
(e)
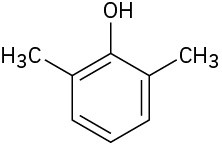
(f)
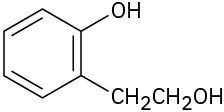
Problem 17-3
Hydrogen-bonding is more difficult in hindered alcohols.
Problem 17-4
(a) HC≡CH < (CH3)2CHOH < CH3OH < (CF3)2CHOH
(b) p-Methylphenol < Phenol < p-(Trifluoromethyl)phenol
(c) Benzyl alcohol < Phenol < p-Hydroxybenzoic acid
Problem 17-5
The electron-withdrawing nitro group stabilizes an alkoxide ion, but the electron-donating methoxyl group destabilizes the anion.
Problem 17-6
(a) 2-Methyl-3-pentanol
(b) 2-Methyl-4-phenyl-2-butanol
(c) meso-5,6-Decanediol
Problem 17-7
(a)NaBH4
(b) LiAlH4
(c) LiAlH4
Problem 17-8
(a) Benzaldehyde or benzoic acid (or ester)
(b) Acetophenone
(c) Cyclohexanone
(d) 2-Methylpropanal or 2-methylpropanoic acid (or ester)
Problem 17-9
(a) 1-Methylcyclopentanol (b) 1,1-Diphenylethanol (c) 3-Methyl-3-hexanol
Problem 17-10
(a)
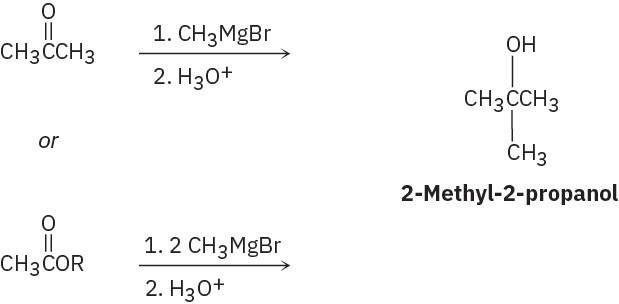
(b)
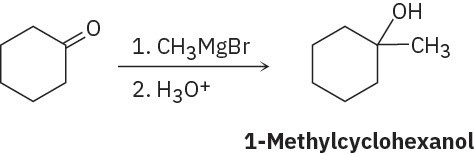
(c)
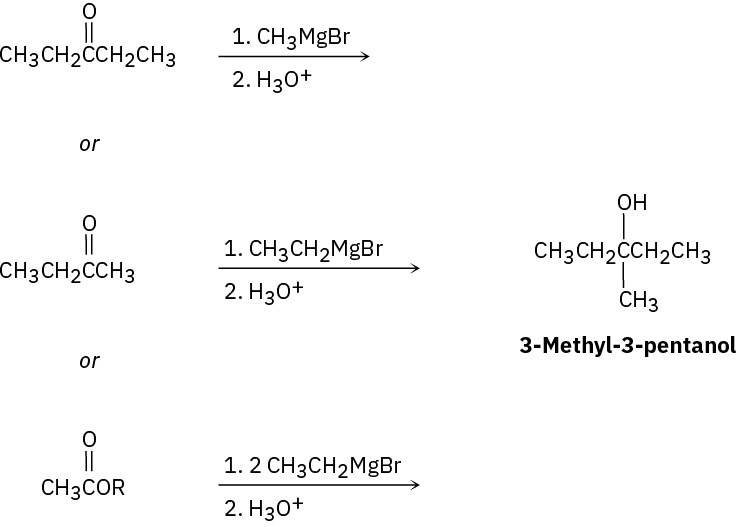
(d)
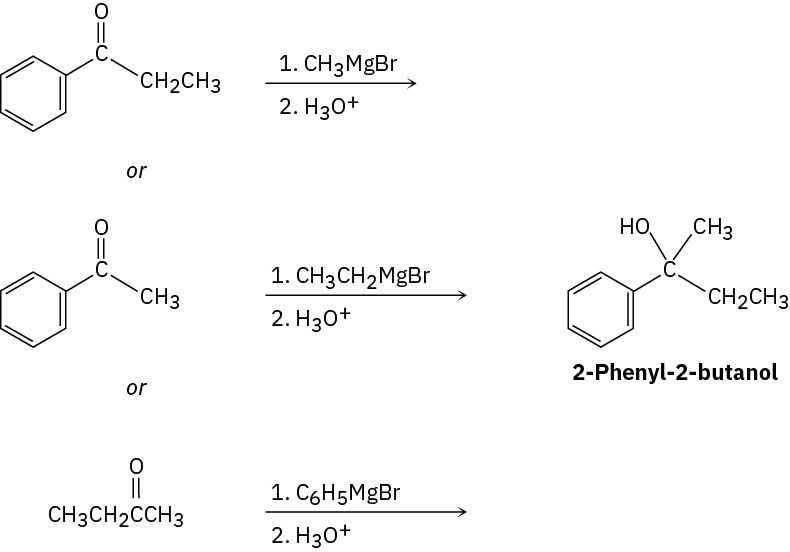
(e)
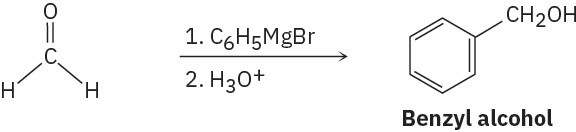
(f)
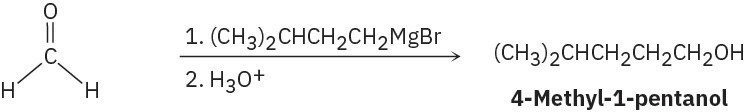
Problem 17-11
Cyclohexanone + CH3CH2MgBr
Problem 17-12
1. p-TosCl, pyridine; 2. NaCN
Problem 17-13
(a) Methyl-2-pentene (b) Methylcyclohexene (c) Methylcyclohexene (d) 2,3-Dimethyl-2-pentene (e) Methyl-2-pentene
Problem 17-14
(a) Phenylethanol (b) Methyl-1-propanol (c) Cyclopentanol
Problem 17-15
(a) Hexanoic acid, hexanal
(b) 2-Hexanone
(c) Hexanoic acid, no reaction
Problem 17-16
SN2 reaction of F− on silicon with displacement of alkoxide ion.
Problem 17-17
Protonation of 2-methylpropene gives the tert-butyl cation, which carries out an electrophilic aromatic substitution reaction.
Problem 17-18
Disappearance of –OH absorption; appearance of C=O
Problem 17-19
(a) Singlet (b) Doublet (c) Triplet (d) Doublet (e) Doublet (f) Singlet
Chapter 18
Problem 18-1
(a) Diisopropyl ether (b) Cyclopentyl propyl ether (c p-Bromoanisole or 4-bromo-1-methoxybenzene (d) Methoxycyclohexene (e) Ethyl isobutyl ether (f Allyl vinyl ether
Problem 18-2
A mixture of diethyl ether, dipropyl ether, and ethyl propyl ether is formed in a 1 : 1 : 2 ratio.
Problem 18-3
(a) CH3CH2CH2O− + CH3Br
(b) PhO− + CH3Br
(c) (CH3)2CHO− + PhCH2Br
(d) (CH3)3CCH2O− + CH3CH2Br
Problem 18-4
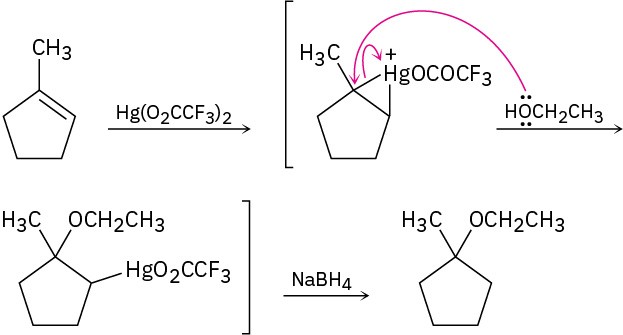
Problem 18-5
(a) Either method (b) Williamson (c) Alkoxymercuration (d) Williamson
Problem 18-6
(a) Bromoethane > 2-Bromopropane > Bromobenzene
(b) Bromoethane > Chloroethane > 1-Iodopropene
Problem 18-7
(a)
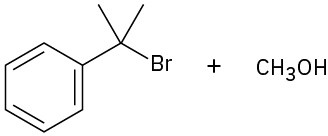
(b)
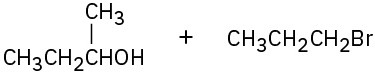
Problem 18-8
Protonation of the oxygen atom, followed by E1 reaction
Problem 18-9
Br− and I− are better nucleophiles than Cl−.
Problem 18-10
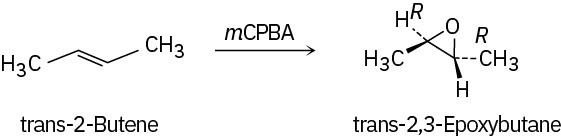
Problem 18-11
Epoxidation of cis-2-butene yields cis-2,3-epoxybutane, while epoxidation of trans-2- butene yields trans-2,3-epoxybutane.
Problem 18-12
(a)
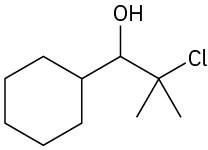
(b)
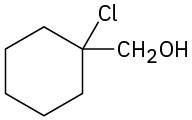
Problem 18-13
(a) 1-Methylcyclohexene + OsO4; then NaHSO3
(b) 1-Methylcyclohexene + m-chloroperoxybenzoic acid, then H3O+
Problem 18-14
(a)
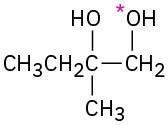
(b)
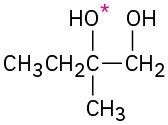
(c)
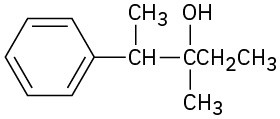
Problem 18-15
Problem 18-16
(a) Butanethiol (b) 2,2,6-Trimethyl-4-heptanethiol (c) 2-Cyclopentene-1-thiol (d) Ethyl isopropyl sulfide (e) Di(methylthio)benzene (f) 3-(Ethylthio)cyclohexanone
Problem 18-17
(a) 1. LiAlH4; 2. PBr3; 3. (𝐻!𝑁)!𝐶═𝑆; 4. H2O, NaOH
(b) 1. HBr; 2. (𝐻!𝑁)!𝐶═𝑆; 3. H2O, NaOH
Problem 18-18
1,2-Epoxybutane
Problem 18-69
Acetyl chloride is more electrophilic than acetone.
Problem 18-70
Problem 18-71
(a) Nucleophilic acyl substitution (b) Nucleophilic addition (c) Carbonyl condensation
Chapter 19
Problem 19-1
(a) Methyl-3-pentanone (b) Phenylpropanal (c) 2,6-Octanedione (d) trans-2-Methylcyclohexanecarbaldehyde (e) Hexenal (f) cis-2,5-Dimethylcyclohexanone
Problem 19-2
(a)
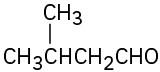
(b)
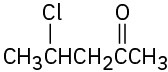
(c)
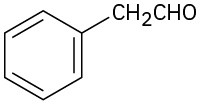
(d)
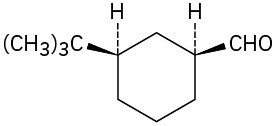
(e)
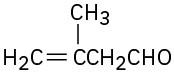
(f)
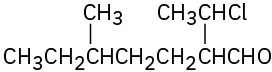
Problem 19-3
(a) Dess–Martin periodinane (b) 1. O3; 2. Zn (c) DIBAH (d) 1. BH3, then H2O2, NaOH; 2. Dess–Martin periodinane
Problem 19-4
(a) HgSO4, H3O+
(b) 1. CH3COCl, AlCl3; 2. Br2, FeBr3
(c) 1. Mg; 2. CH3CHO; 3. H3O+; 4. CrO3
(d) 1. BH3; 2. H2O2, NaOH; 3. CrO3
Problem 19-5
Problem 19-6
The electron-withdrawing nitro group in p-nitrobenzaldehyde polarizes the carbonyl group.
Problem 19-7
CCl3CH(OH)2
Problem 19-8
Labeled water adds reversibly to the carbonyl group.
Problem 19-9
The equilibrium is unfavorable for sterically hindered ketones.
Problem 19-10
Problem 19-11
The steps are the exact reverse of the forward reaction shown in Figure 19.7.
Problem 19-12
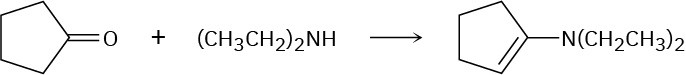
Problem 19-13
(a) H2/Pd (b) N2H4, KOH (c) 1. H2/Pd; 2. N2H4, KOH
Problem 19-14
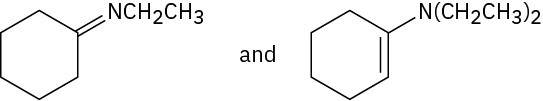
The mechanism is identical to that between a ketone and 2 equivalents of a monoalcohol, shown in Figure 19.11.
Problem 19-15
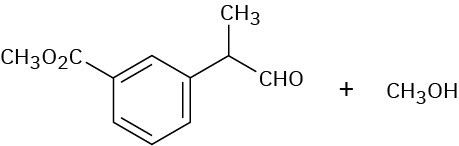
Problem 19-16
(a) Cyclohexanone + (Ph)3P=CHCH3
(b) Cyclohexanecarbaldehyde + (Ph)3P=CH2
(c) Acetone + (Ph)3P=CHCH2CH2CH3
(d) Acetone + (Ph)3P=CHPh
(e) PhCOCH3 + (Ph)3P=CHPh
(f) 2-Cyclohexenone + (Ph)3P=CH2
Problem 19-17
Problem 19-18
Intramolecular Cannizzaro reaction
Problem 19-19
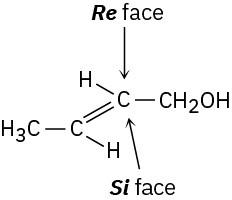
Addition of the pro-R hydrogen of NADH takes place on the Re face of pyruvate.
Problem 19-20
The −OH group adds to the Re face at C2, and −H adds to the Re face at C3, to yield (2R,3S)- isocitrate.
Problem 19-21
Problem 19-22
(a) 3-Buten-2-one + (CH3CH2CH2)2CuLi (b) 3-Methyl-2-cyclohexenone + (CH3)2CuLi (c) 4-tert-Butyl-2-cyclohexenone + (CH3CH2)2CuLi (d) Unsaturated ketone + (H2C=CH)2CuLi
Problem 19-23
Look for appearance of either an alcohol or a saturated ketone in the product.
Problem 19-24
(a) 1715 cm−1
(b) 1685 cm−1
(c) 1750 cm−1
(d) 1705 cm−1
(e) 1715 cm−1
(f) 1705 cm−1
Problem 19-25
(a) Different peaks due to McLafferty rearrangement
(b) Different peaks due to α cleavage and McLafferty rearrangement
(c) Different peaks due to McLafferty rearrangement
Problem 19-26
IR: 1750 cm−1; MS: 140, 84
Chapter 20
Problem 20-1
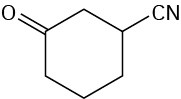
(a)Methylbutanoic acid (b) Bromopentanoic acid (c) 2-Ethylpentanoic acid (d) cis-4-Hexenoic acid (e) 2,4-Dimethylpentanenitrile (f) cis-1,3-Cyclopentanedicarboxylic acid
Problem 20-2
(a)
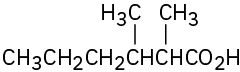
(b)
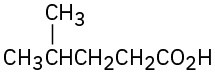
(c)
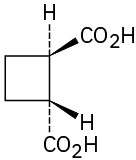
(d)
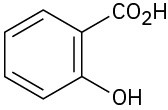
(e)

(f)

Problem 20-3
Dissolve the mixture in ether, extract with aqueous NaOH, separate and acidify the aqueous layer, and extract with ether.
Problem 20-4
43%
Problem 20-5
(a) 82% dissociation (b) 73% dissociation
Problem 20-6
Lactic acid is stronger because of the inductive effect of the −OH group.
Problem 20-7
The dianion is destabilized by repulsion between charges.
Problem 20-8
More reactive
Problem 20-9
(a) p-Methylbenzoic acid < Benzoic acid < p-Chlorobenzoic acid
(b) Acetic acid < Benzoic acid < p-Nitrobenzoic acid
Problem 20-10
(a) 1. Mg; 2. CO2; 3. H3O+
(b) 1. Mg; 2. CO2; 3. H3O+ or 1. NaCN; 2. H3O+
Problem 20-11
1. NaCN; 2. H3O+; 3. LiAlH4
Problem 20-12
1. PBr3; 2. NaCN; 3. H3O+; 4. LiAlH4
Problem 20-13
(a) Propanenitrile + CH3CH2MgBr, then H3O+ (b) p-Chlorobenzonitrile + CH3MgBr, then H3O+
Problem 20-14
1. NaCN; 2. CH3CH2MgBr, then H3O+
Problem 20-15
A carboxylic acid has a very broad −OH absorption at 2500–3300 cm−1.
Problem 20-16
4-Hydroxycyclohexanone: H–C–O absorption near 4 δ in the 1H spectrum and C═O absorption near 210 δ in the 13C spectrum. Cyclopentanecarboxylic acid: –CO2H absorption near 12 δ in the 1H spectrum and –CO2H absorption near 170 δ in the 13C spectrum.
Chapter 21
Problem 21-1
(a) Methylpentanoyl chloride
(b) Cyclohexylacetamide
(c) Isopropyl 2-methylpropanoate
(d) Benzoic anhydride
(e) Isopropyl cyclopentanecarboxylate
(f) Cyclopentyl 2-methylpropanoate
(g) N-Methyl-4-pentenamide
(h) (R)-2-Hydroxypropanoyl phosphate
(i) Ethyl 2,3-dimethyl-2-butenethioate
Problem 21-2
(a)
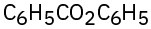
(b)

(c)
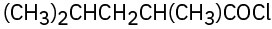
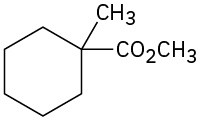
(d)
(e)
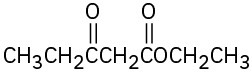
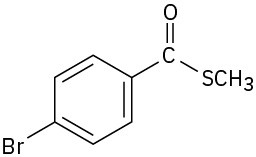
(f)
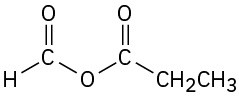
(g)
(h)
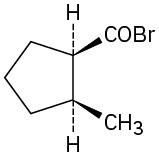
Problem 21-3
Problem 21-4
(a) Acetyl chloride > Methyl acetate > Acetamide
(b) Hexafluoroisopropyl acetate > 2,2,2-Trichloroethyl acetate > Ethyl acetate
Problem 21-5
(a) CH3CO2– Na+
(b) CH3CONH2
(c) CH3CO2CH3 + CH3CO2– Na+
(d) CH3CONHCH3
Problem 21-6
Problem 21-7
(a) Acetic acid + 1-butanol
(b) Butanoic acid + methanol
(c) Cyclopentanecarboxylic acid + isopropyl alcohol
Problem 21-8
Problem 21-9
(a) Propanoyl chloride + methanol (b) Acetyl chloride + ethanol (c) Benzoyl chloride + ethanol
Problem 21-10
Benzoyl chloride + cyclohexanol
Problem 21-11
This is a typical nucleophilic acyl substitution reaction, with morpholine as the nucleophile and chloride as the leaving group.
Problem 21-12
(a) Propanoyl chloride + methylamine (b) Benzoyl chloride + diethylamine (c) Propanoyl chloride + ammonia
Problem 21-13
(a) Benzoyl chloride + [(CH3)2CH]2CuLi, or 2-methylpropanoyl chloride + Ph2CuLi
(b)
Propenoyl chloride + (CH3CH2CH2)2CuLi, or butanoyl chloride + (H2C=CH)2CuLi
Problem 21-14
This is a typical nucleophilic acyl substitution reaction, with p-hydroxyaniline as the nucleophile and acetate ion as the leaving group.
Problem 21-15
Monomethyl ester of benzene-1,2-dicarboxylic acid
Problem 21-16
Reaction of a carboxylic acid with an alkoxide ion gives the carboxylate ion.
Problem 21-17
LiAlH4 gives HOCH2CH2CH2CH2OH; DIBAH gives HOCH2CH2CH2CHO.
Problem 21-18
(a) CH3CH2CH2CH(CH3)CH2OH + CH3OH (b) PhOH + PhCH2OH
Problem 21-19
(a) Ethyl benzoate + 2 CH3MgBr (b) Ethyl acetate + 2 PhMgBr (c) Ethyl pentanoate + 2 CH3CH2MgBr
Problem 21-20
(a) H2O, NaOH
(b) Benzoic acid + LiAlH4
(c) LiAlH4
Problem 21-21
1. Mg; 2. CO2, then H3O+; 3. SOCl2; 4. (CH3)2NH; 5. LiAlH4
Problem 21-22
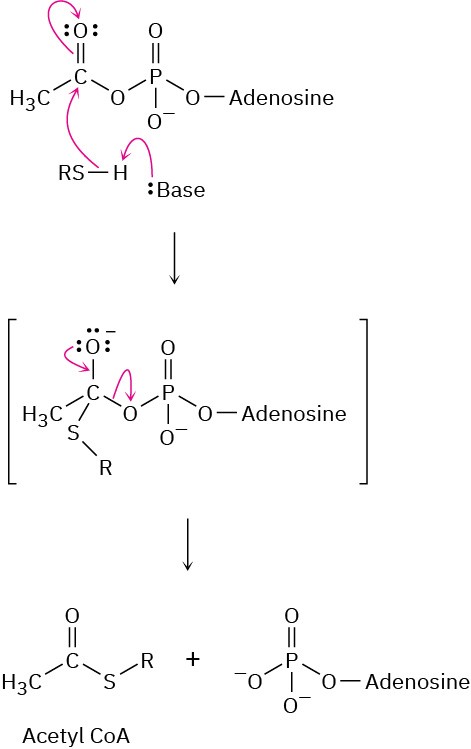
Problem 21-23
(a)
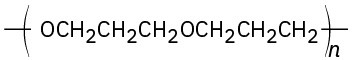
(b)
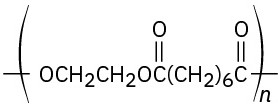
(c)
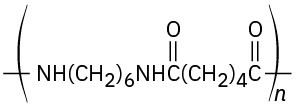
Problem 21-24
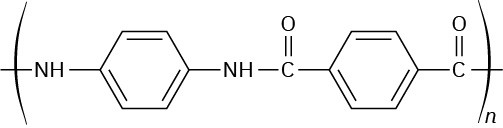
Problem 21-25
(a) Ester
(b) Acid chloride
(c) Carboxylic acid
(d) Aliphatic ketone or cyclohexanone
Problem 21-26
(a) CH3CH2CH2CO2CH2CH3 and other possibilities
(b) CH3CON(CH3)2
(c) CH3CH=CHCOCl or H2C=C(CH3)COCl
Chapter 22
Problem 22-1
(a)
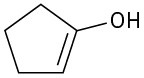
(b)
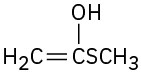
(c)
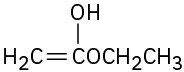
(d)
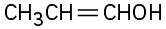
(e)
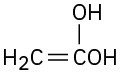
(f)
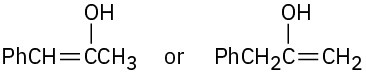
Problem 22-2
(a) 4(b) 3(c) 3(d) 2(e) 4(f) 5
Problem 22-3
Problem 22-4
Acid-catalyzed formation of an enol is followed by deuteronation of the enol double bond and dedeuteronation of oxygen.
Problem 22-5
1. Br2; 2. Pyridine, heat
Problem 22-6
The intermediate α-bromo acid bromide undergoes a nucleophilic acyl substitution reaction with methanol to give an α-bromo ester.
Problem 22-7
(a) CH3CH2CHO
(b) (CH3)3CCOCH3
(c) CH3CO2H
(d) PhCONH2
(e) CH3CH2CH2CN
(f) CH3CON(CH3)2
Problem 22-8
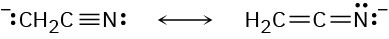
Problem 22-9
Acid is regenerated, but base is used stoichiometrically.
Problem 22-10
(a) 1. Na+ −OEt; 2. PhCH2Br; 3. H3O+
(b) 1. Na+ −OEt; 2. CH3CH2CH2Br; 3. Na+ −OEt; 4. CH3Br; 5. H3O+
(c) 1. Na+ −OEt; 2. (CH3)2CHCH2Br; 3. H3O+
Problem 22-11
Malonic ester has only two acidic hydrogens to be replaced.
Problem 22-12
1. Na+ −OEt; 2. (CH3)2CHCH2Br; 3. Na+ −OEt; 4. CH3Br; 5. H3O+
Problem 22-13
(a) (CH3)2CHCH2Br
(b) PhCH2CH2Br
Problem 22-14
None can be prepared.
Problem 22-15
1. 2 Na+ −OEt; 2. BrCH2CH2CH2CH2Br; 3. H3O+
Problem 22-16
(a) Alkylate phenylacetone with CH3I
(b) Alkylate pentanenitrile with CH3CH2I
(c) Alkylate cyclohexanone with H2C═CHCH2Br
(d) Alkylate cyclohexanone with excess CH3I
(e) Alkylate C6H5COCH2CH3 with CH3I
(f) Alkylate methyl 3-methylbutanoate with CH3CH2I
Chapter 23
Problem 23-1
(a)
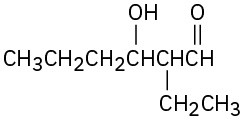
(b)
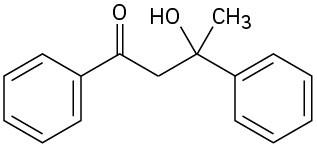
(c)
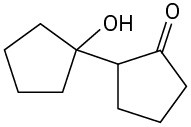
Problem 23-2
The reverse reaction is the exact opposite of the forward reaction shown in Figure 23.2.
Problem 23-3 (a)
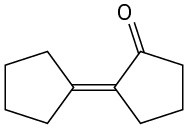
(b)
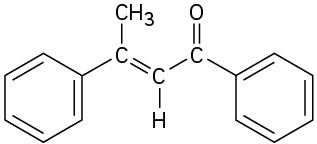
(c)
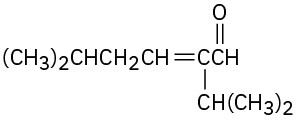
Problem 23-4
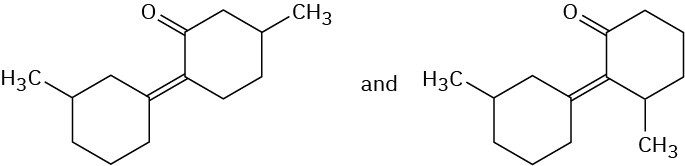
Problem 23-5
(a) Not an aldol product
(b) Pentanone
Problem 23-6
1. NaOH; 2. LiAlH4; 3. H2/Pd
Problem 23-7
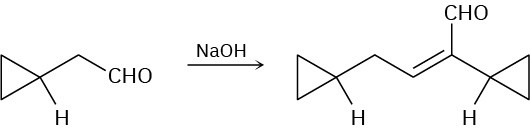
Problem 23-8
(a) C6H5CHO + CH3COCH3
(b) Not easily prepared
(c) Not easily prepared
Problem 23-9
The CH2 position between the two carbonyl groups is so acidic that it is completely deprotonated to give a stable enolate ion.
Problem 23-10
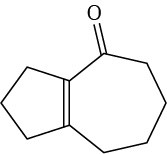
Problem 23-11
(a)
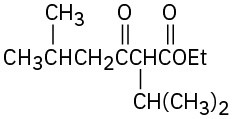
(b)
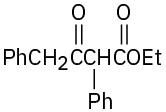
(c)
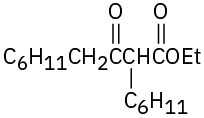
Problem 23-12
The cleavage reaction is the exact reverse of the forward reaction.
Problem 23-13
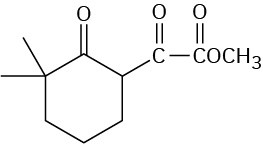
Problem 23-14
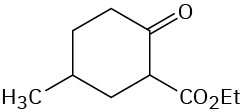
Problem 23-15
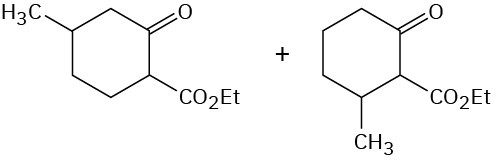
Problem 23-16
(a)
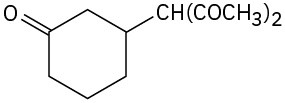
(b)

(c)
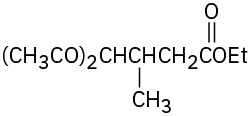
Problem 23-17
(a)
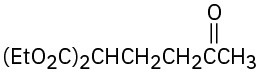
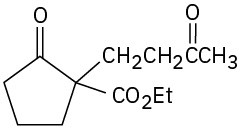
(b)
Problem 23-18
CH3CH2COCH═CH2 + CH3CH2NO2
Problem 23-19
(a)
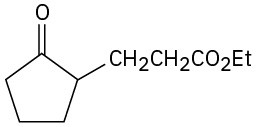
(b)
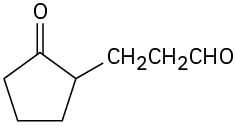
(c)
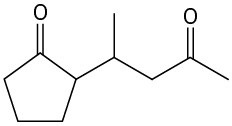
Problem 23-20
(a) Cyclopentanone enamine + propenenitrile
(b) Cyclohexanone enamine + methyl propenoate
Problem 23-21
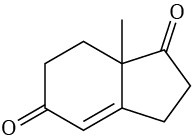
Problem 23-22
2,5,5-Trimethyl-1,3-cyclohexanedione + 1-penten-3-one
Chapter 24
Problem 24-1
(a) N-Methylethylamine
(b) Tricyclohexylamine
(c) N-Ethyl-N-methylcyclohexylamine
(d) N-Methylpyrrolidine
(e) Diisopropylamine
(f) 1,3-Butanediamine
Problem 24-2
(a)
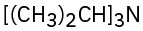
(b)

(c)
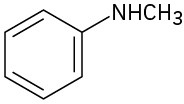
(d)
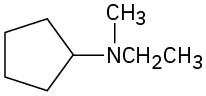
(e)
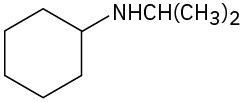
(f)
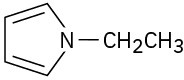
Problem 24-3 (a)
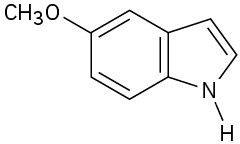
(b)
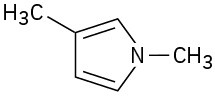
(c)
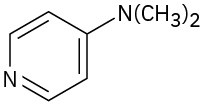
(d)
Problem 24-4
(a) CH3CH2NH2
(b) NaOH
(c) CH3NHCH3
Problem 24-5
Propylamine is stronger; benzylamine pKb = 4.67; propylamine pKb = 3.29
Problem 24-6
(a) Nitroaniline < p-Aminobenzaldehyde < p-Bromoaniline
(b) p-Aminoacetophenone < p-Chloroaniline < p-Methylaniline
(c) p-(Trifluoromethyl)aniline < p-(Fluoromethyl)aniline < p-Methylaniline
Problem 24-7
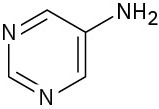
Pyrimidine is essentially 100% neutral (unprotonated).
Problem 24-8
(a) Propanenitrile or propanamide
(b) N-Propylpropanamide
(c) Benzonitrile or benzamide
(d) N-Phenylacetamide
Problem 24-9
The reaction takes place by two nucleophilic acyl substitution reactions.
Problem 24-10
Problem 24-11
(a) Ethylamine + acetone, or isopropylamine + acetaldehyde
(b) Aniline + acetaldehyde
(c) Cyclopentylamine + formaldehyde, or methylamine + cyclopentanone
Problem 24-12
Problem 24-13
(a) 4,4-Dimethylpentanamide or 4,4-dimethylpentanoyl azide
(b) p-Methylbenzamide or p-methylbenzoyl azide
Problem 24-14
(a) 3-Octene and 4-octene (b) Cyclohexene (c) 3-Heptene (d) Ethylene and cyclohexene
Problem 24-15
H2C═CHCH2CH2CH2N(CH3)2
Problem 24-16
1. HNO3, H2SO4; 2. H2/PtO2; 3. (CH3CO)2O; 4. HOSO2Cl; 5. aminothiazole; 6. H2O, NaOH
Problem 24-17
(a) 1. HNO3, H2SO4; 2. H2/PtO2; 3. 2 CH3Br
(b) 1. HNO3, H2SO4; 2. H2/PtO2; 3. (CH3CO)2O; 4. Cl2; 5. H2O, NaOH
(c) 1. HNO3, H2SO4; 2. Cl2, FeCl3; 3. SnCl2
(d) 1. HNO3, H2SO4; 2. H2/PtO2; 3. (CH3CO)2O; 4. 2 CH3Cl, AlCl3; 5. H2O, NaOH
Problem 24-18
(a) 1. CH3Cl, AlCl3; 2. HNO3, H2SO4; 3. SnCl2; 4. NaNO2, H2SO4; 5. CuBr; 6. KMnO4, H2O
(b) 1. HNO3, H2SO4; 2. Br2, FeBr3; 3. SnCl2, H3O+; 4. NaNO2, H2SO4; 5. CuCN; 6. H3O+
(c) 1. HNO3, H2SO4; 2. Cl2, FeCl3; 3. SnCl2; 4. NaNO2, H2SO4; 5. CuBr
(d) 1. CH3Cl, AlCl3; 2. HNO3, H2SO4; 3. SnCl2; 4. NaNO2, H2SO4; 5. CuCN; 6. H3O+
(e) 1. HNO3, H2SO4; 2. H2/PtO2; 3. (CH3CO)2O; 4. 2 Br2; 5. H2O, NaOH; 6. NaNO2, H2SO4; 7. CuBr
Problem 24-19
1. HNO3, H2SO4; 2. SnCl2; 3a. 2 equiv. CH3I; 3b. NaNO2, H2SO4; 4. product of 3a + product of 3b
Problem 24-20
Problem 24-21
% protonated
Problem 24-22
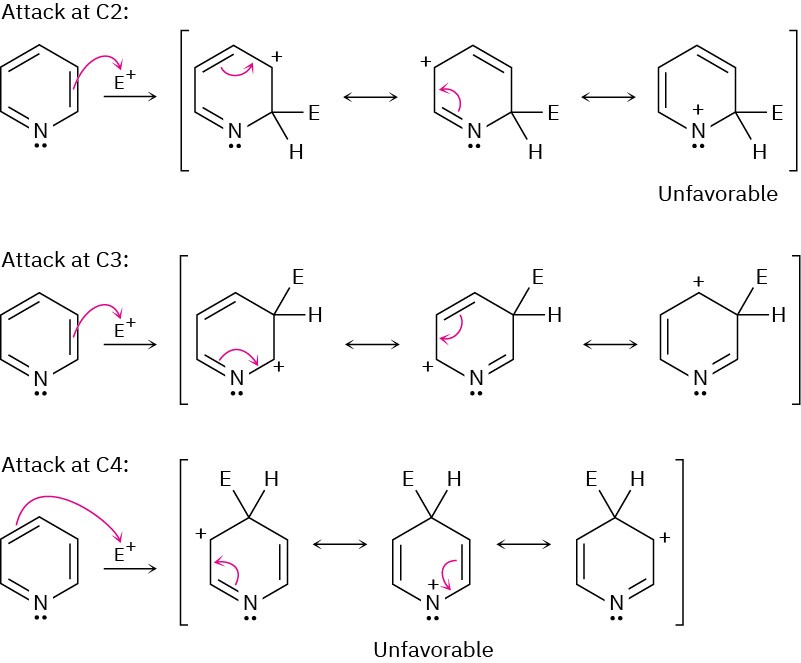
Problem 24-23
The side-chain nitrogen is more basic than the ring nitrogen.
Problem 24-24
Reaction at C2 is disfavored because the aromaticity of the benzene ring is lost.
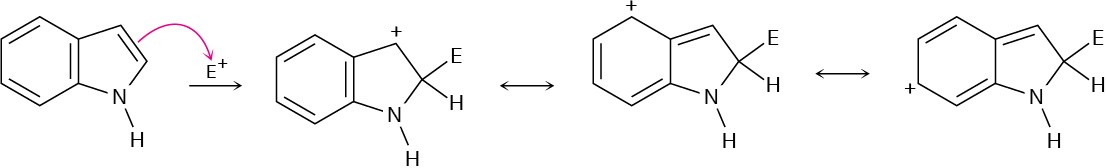
Problem 24-25
(CH3)3CCOCH3 → (CH3)3CCH(NH2)CH3
Chapter 25
Problem 25-1 (a) Aldotetrose (b) Ketopentose (c) Ketohexose (d) Aldopentose
Problem 25-2
(a) S (b) R (c) S
Problem 25-3
A, B, and C are the same.
Problem 25-4
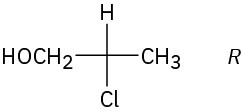
Problem 25-5
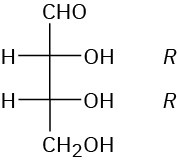
Problem 25-6
(a) L-Erythrose; 2S,3S (b) D-Xylose; 2R,3S,4R (c) D-Xylulose; 3S,4R
Problem 25-7
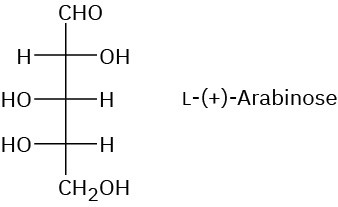
Problem 25-8
(a)
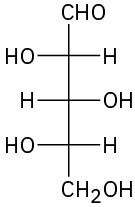
(b)
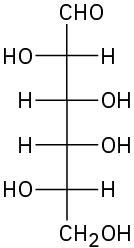
(c)
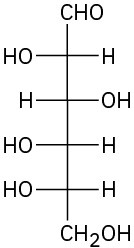
Problem 25-9
16 D and 16 L aldoheptoses
Problem 25-10
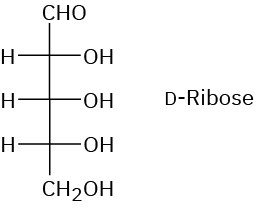
Problem 25-11
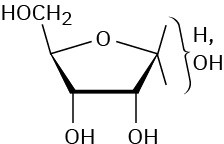
Problem 25-12
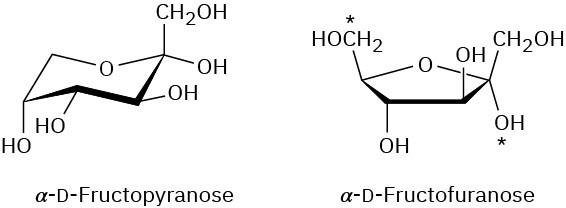
Problem 25-13
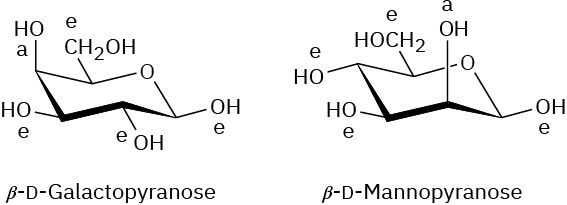
Problem 25-14
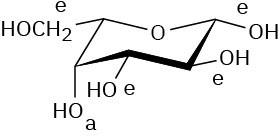
Problem 25-15
α-D-Allopyranose
Problem 25-16
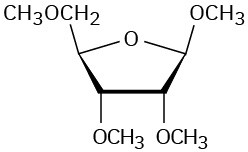
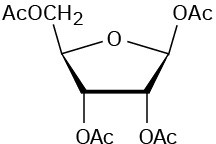
Problem 25-17
D-Galactitol has a plane of symmetry and is a meso compound, whereas D-glucitol is chiral.
Problem 25-18
The −CHO end of L-gulose corresponds to the −CH2OH end of D-glucose after reduction.
Problem 25-19
D-Allaric acid has a symmetry plane and is a meso compound, but D-glucaric acid is chiral.
Problem 25-20
D-Allose and D-galactose yield meso aldaric acids; the other six D-hexoses yield optically active aldaric acids.
Problem 25-21
D-Allose + D-altrose
Problem 25-22
L-Xylose
Problem 25-23
D-Xylose and D-lyxose
Problem 25-24
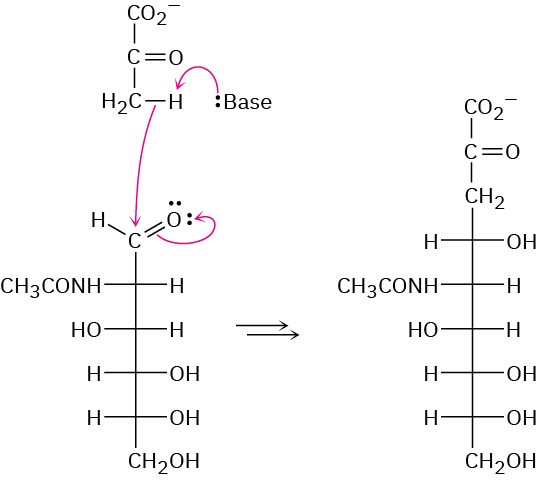
Problem 25-25
(a) The hemiacetal ring is reduced. (b) The hemiacetal ring is oxidized. (c) All hydroxyl groups are acetylated.
Chapter 26
Problem 26-1
Aromatic: Phe, Tyr, Trp, His; sulfur-containing: Cys, Met; alcohols: Ser, Thr; hydrocarbon side chains: Ala, Ile, Leu, Val, Phe
Problem 26-2
The sulfur atom in the −CH2SH group of cysteine makes the side chain higher in ranking than the −CO2H group.
Problem 26-3
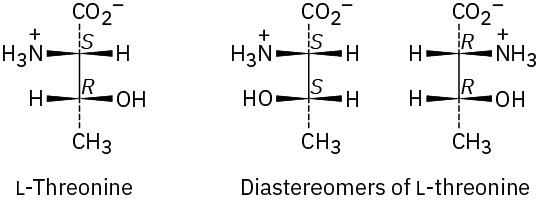
Problem 26-4
Net positive at pH = 5.3; net negative at pH = 7.3
Problem 26-5
(a) Start with 3-phenylpropanoic acid: 1. Br2, PBr3; 2. NH3
(b) Start with 3-methylbutanoic acid: 1. Br2, PBr3; 2. NH3
Problem 26-6
(a)

(b)
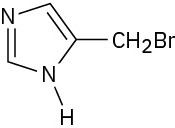
(c)
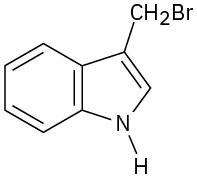
(d)
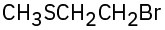
Problem 26-7
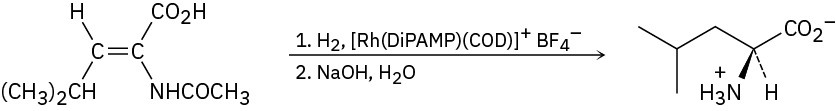
Problem 26-8
Val-Tyr-Gly (VYG), Tyr-Gly-Val (YGV), Gly-Val-Tyr (GVY), Val-Gly-Tyr (VGY), Tyr-Val-Gly (YVG), Gly-Tyr-Val (GYV)
Problem 26-9
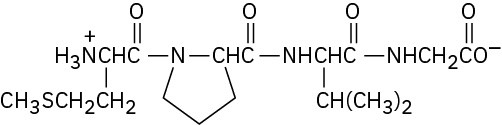
Problem 26-10
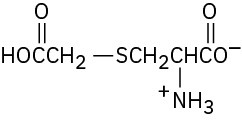
Problem 26-11
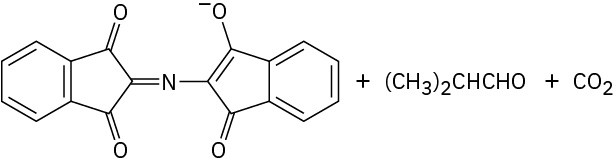
Problem 26-12
Trypsin: Asp-Arg + Val-Tyr-Ile-His-Pro-Phe Chymotrypsin: Asp-Arg-Val-Tyr + Ile-His-Pro-Phe
Problem 26-13
Methionine
Problem 26-14
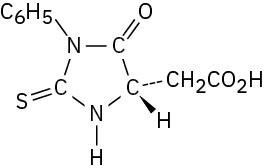
Problem 26-15
(a) Arg-Pro-Leu-Gly-Ile-Val (b) Val-Met-Trp-Asp-Val-Leu (VMWNVL)
Problem 26-16
This is a typical nucleophilic acyl substitution reaction, with the amine of the amino acid as the nucleophile and tert-butyl carbonate as the leaving group. The tert-butyl carbonate then loses CO2 and gives tert-butoxide, which is protonated.
Problem 26-17
Protect the amino group of leucine.
Protect the carboxylic acid group of alanine.
Couple the protected amino acids with DCC.
Remove the leucine protecting group.
Remove the alanine protecting group.
Problem 26-18
(a) Lyase (b) Hydrolase (c) Oxidoreductase
Chapter 27
Problem 27-1
CH3(CH2)18CO2CH2(CH2)30CH3
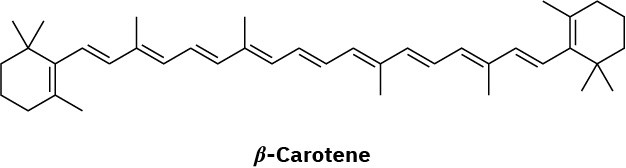
Problem 27-2
Glyceryl tripalmitate is higher melting.
Problem 27-3
[CH3(CH2)7CH═CH(CH2)7CO2−]2 Mg2+
Problem 27-4
Glyceryl dioleate monopalmitate → glycerol + 2 sodium oleate + sodium palmitate
Problem 27-5
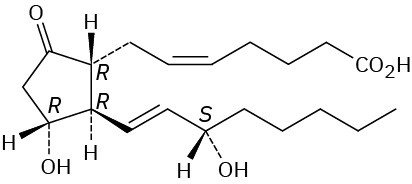
Problem 27-6
The pro-S hydrogen is cis to the −CH3 group; the pro-R hydrogen is trans.
Problem 27-7
(a)
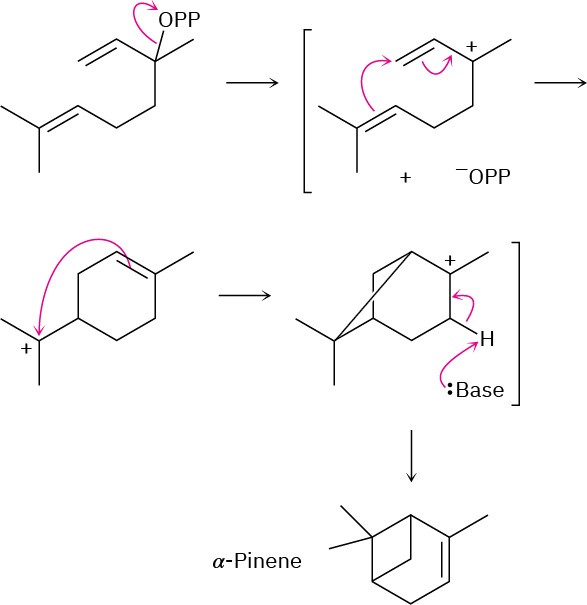
(b)
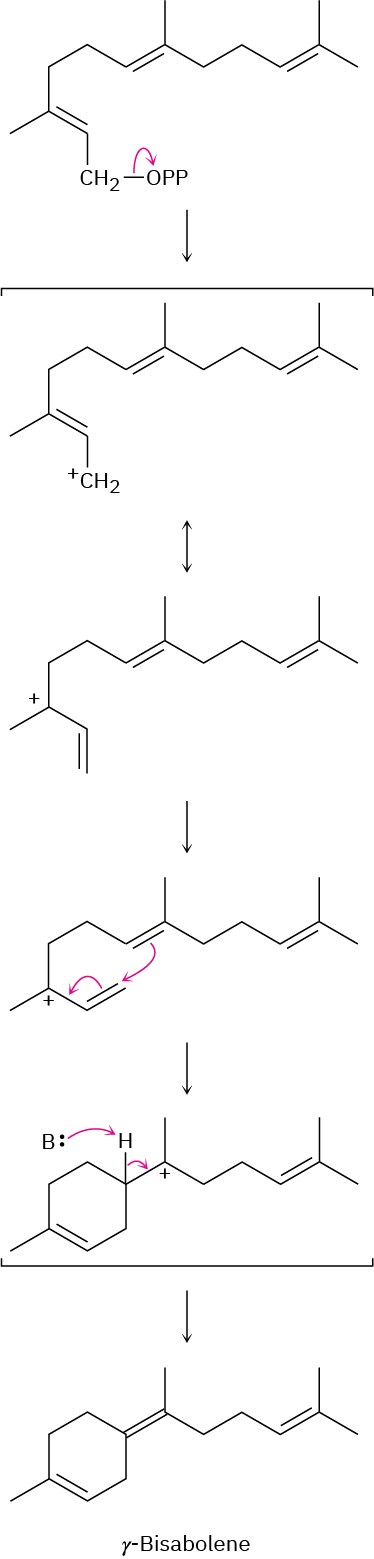
Problem 27-8
(a)
(b)
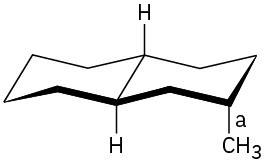
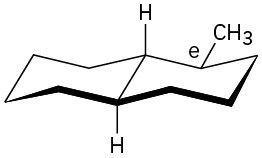
Problem 27-9
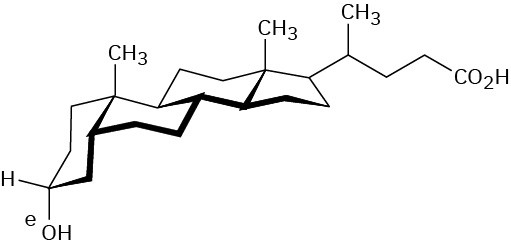
Problem 27-10
Three methyl groups are removed, the side-chain double bond is reduced, and the double bond in the B ring is migrated.
Chapter 28
Problem 28-1
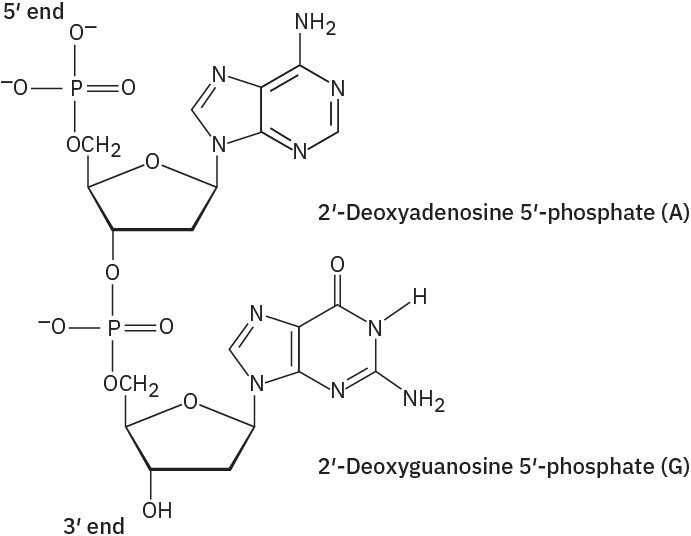
Problem 28-2
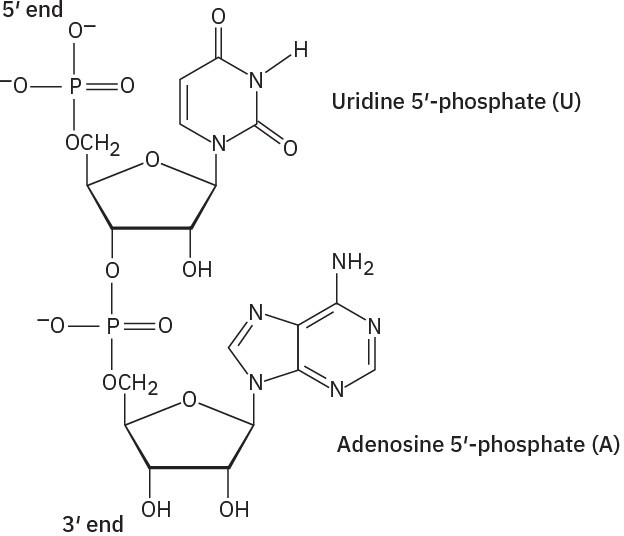
Problem 28-3
(5′) ACGGATTAGCC (3′)
Problem 28-4
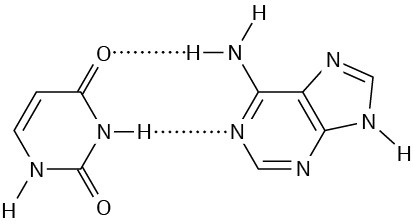
Problem 28-5
(3′) CUAAUGGCAU (5′)
Problem 28-6
(5′) ACTCTGCGAA (3′)
Problem 28-7
(a) GCU, GCC, GCA, GCG
(b) UUU, UUC
(c) UUA, UUG, CUU, CUC, CUA, CUG
(d) UAU, UAC
Problem 28-8
(a) AGC, GGC, UGC, CGC
(b) AAA, GAA
(c) UAA, CAA, GAA, GAG, UAG, CAG
(d) AUA, GUA
Problem 28-9
Leu-Met-Ala-Trp-Pro-Stop
Problem 28-10
(5′) TTA-GGG-CCA-AGC-CAT-AAG (3′)
Problem 28-11
The cleavage is an SN1 reaction that occurs by protonation of the oxygen atom followed by loss of the stable triarylmethyl carbocation.
Problem 28-12
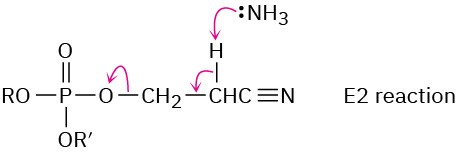
Chapter 29
Problem 29-1
HOCH2CH(OH)CH2OH + ATP → HOCH2CH(OH)CH2OPO32− + ADP
Problem 29-2
Caprylyl CoA → Hexanoyl CoA → Butyryl CoA → 2 Acetyl CoA
Problem 29-3
(a) 8 acetyl CoA; 7 passages (b) 10 acetyl CoA; 9 passages
Problem 29-4
The dehydration is an E1cB reaction.
Problem 29-5
At C2, C4, C6, C8, and so forth
Problem 29-6
The Si face
Problem 29-7
Steps 7 and 10
Problem 29-8
Steps 1, 3: Phosphate transfers; steps 2, 5, 8: isomerizations; step 4: retro-aldol reaction; step 5: oxidation and nucleophilic acyl substitution; steps 7, 10: phosphate transfers; step 9: E1cB dehydration
Problem 29-9
C1 and C6 of glucose become –CH3 groups; C3 and C4 become CO2.
Problem 29-10
Citrate and isocitrate
Problem 29-11
E1cB elimination of water, followed by conjugate addition
Problem 29-12
pro-R; anti geometry
Problem 29-13
The reaction occurs by two sequential nucleophilic acyl substitutions, the first by a cysteine residue in the enzyme, with phosphate as leaving group, and the second by hydride donation from NADH, with the cysteine residue as leaving group.
Problem 29-14
Initial imine formation between PMP and α-ketoglutarate is followed by double-bond rearrangement to an isomeric imine and hydrolysis.
Problem 29-15
(CH3)2CHCH2COCO2−
Problem 29-16
Asparagine
Chapter 30
Problem 30-1
Ethylene: ψ1 is the HOMO and ψ2* is the LUMO in the ground state; ψ2* is the HOMO and there is no LUMO in the excited state. 1,3-Butadiene: ψ2 is the HOMO and ψ3* is the LUMO in the ground state; ψ3* is the HOMO and ψ4* is the LUMO in the excited state.
Problem 30-2
Disrotatory: cis-5,6-dimethyl-1,3-cyclohexadiene;
conrotatory: trans-5,6-dimethyl-1,3-cyclohexadiene. Disrotatory closure occurs.
Problem 30-3
The more stable of two allowed products is formed.
Problem 30-4
trans-5,6-Dimethyl-1,3-cyclohexadiene; cis-5,6-dimethyl-1,3-cyclohexadiene
Problem 30-5
cis-3,6-Dimethylcyclohexene; trans-3,6-dimethylcyclohexene
Problem 30-6
A [6 + 4] suprafacial cycloaddition
Problem 30-7
An antarafacial [1,7] sigmatropic rearrangement
Problem 30-8

Problem 30-9
A series of [1,5] hydrogen shifts occur.
Problem 30-10
o-(1-Methylallyl)phenol
Problem 30-11
Claisen rearrangement is followed by a Cope rearrangement.
Problem 30-12
(a) Conrotatory (b) Disrotatory (c) Suprafacial (d) Antarafacial (e) Suprafacial
Chapter 31
Problem 31-1
H2C═CHCO2CH3 < H2C═CHCl < H2C═CHCH3 < H2C═CH–C6H5
Problem 31-2
H2C═CHCH3 < H2C═CHC6H5 < H2C═CHC≡N
Problem 31-3
The intermediate is a resonance-stabilized benzylic carbanion, 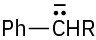
Problem 31-4
The polymer has no chirality centers.
Problem 31-5
The polymers are racemic and have no optical rotation.
Problem 31-6
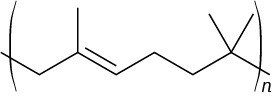
Problem 31-7
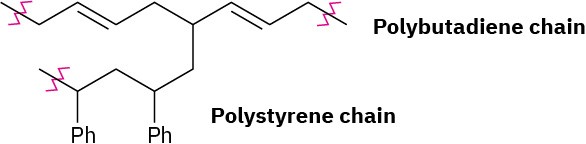
Problem 31-8
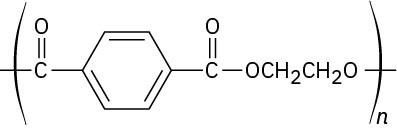
Problem 31-9
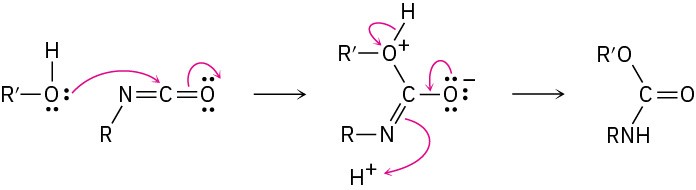
Problem 31-10
Vestenamer: ADMET polymerization of 1,9-decadiene or ROMP of cyclooctene; Norsorex: ROMP of norbornene.
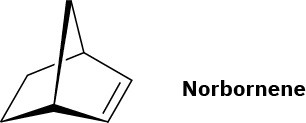
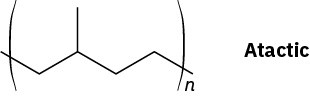
Problem 31-11
Problem 31-12