Visualizing Chemistry
Problem 28-13
Identify the following bases, and tell whether each is found in DNA, RNA, or both:
(a)
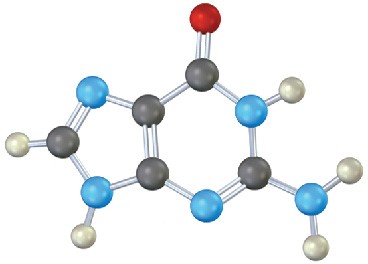
(b)
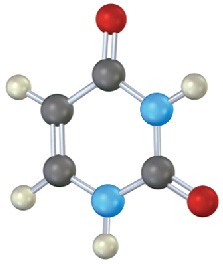
(c)
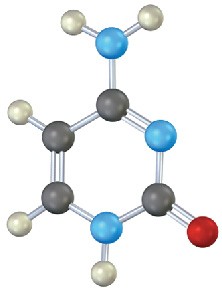
Problem 28-14
Identify the following nucleotide, and tell how it is used:
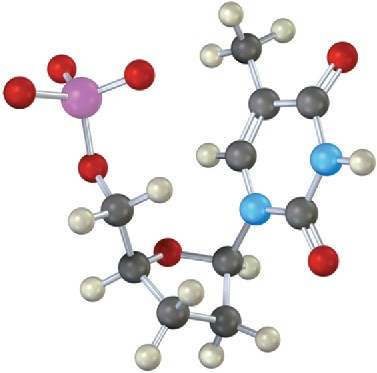
Problem 28-15
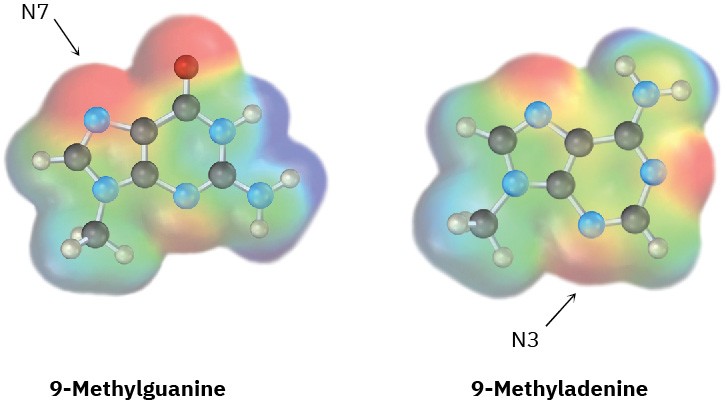
Amine bases in nucleic acids can react with alkylating agents in typical SN2 reactions. Look at the following electrostatic potential maps, and tell which is the better nucleophile, guanine or adenine. The reactive positions in each are indicated.
Mechanism Problems
Problem 28-16
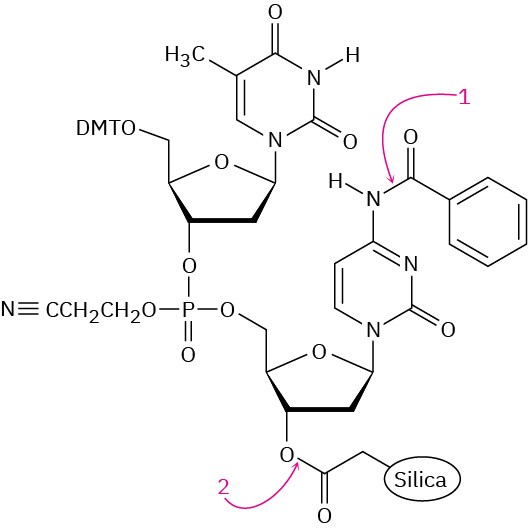
The final step in DNA synthesis is deprotection by treatment with aqueous ammonia. Show the mechanisms by which deprotection occurs at the points indicated in the following structure:
Problem 28-17
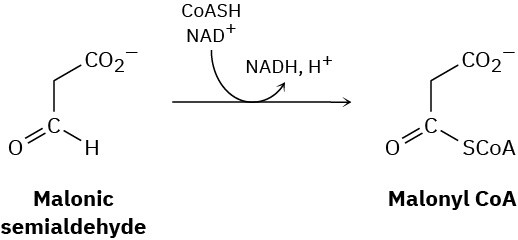
The final step in the metabolic degradation of uracil is the oxidation of malonic semialdehyde to give malonyl CoA. Propose a mechanism.
Problem 28-18
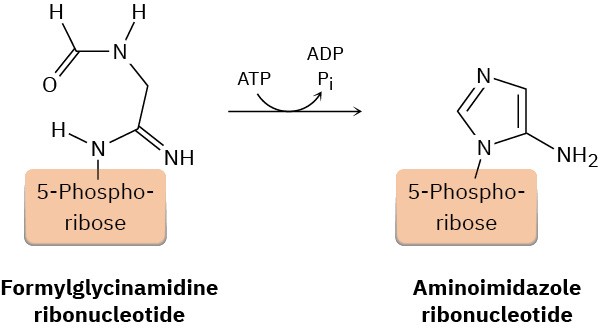
One of the steps in the biosynthesis of a nucleotide called inosine monophosphate is the formation of aminoimidazole ribonucleotide from formylglycinamidine ribonucleotide. Propose a mechanism.
Problem 28-19
One of the steps in the metabolic degradation of guanine is hydrolysis to give xanthine. Propose a mechanism.
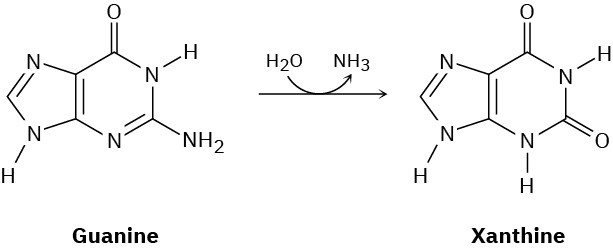
Problem 28-20
One of the steps in the biosynthesis of uridine monophosphate is the reaction of aspartate with carbamoyl phosphate to give carbamoyl aspartate followed by cyclization to form dihydroorotate. Propose mechanisms for both steps.
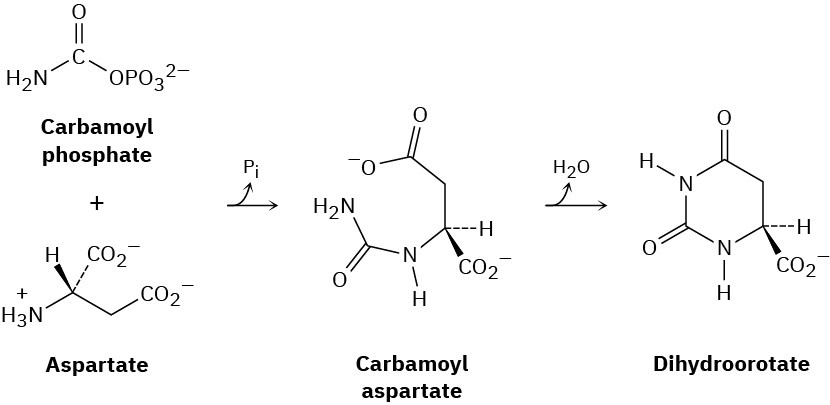
General Problems
Problem 28-21
Human brain natriuretic peptide (BNP) is a small peptide of 32 amino acids used in the treatment of congestive heart failure. How many nitrogen bases are present in the DNA that codes for BNP?
Problem 28-22
Human and horse insulin both have two polypeptide chains, with one chain containing 21 amino acids and the other containing 30 amino acids. They differ in primary structure at two places. At position 9 in one chain, human insulin has Ser and horse insulin has Gly; at position 30 in the other chain, human insulin has Thr and horse insulin has Ala. How must the DNA for the two insulins differ?
Problem 28-23
The DNA of sea urchins contains about 32% A. What percentages of the other three bases would you expect in sea urchin DNA? Explain.
Problem 28-24
The codon UAA stops protein synthesis. Why does the sequence UAA in the following stretch of mRNA not cause any problems?
-GCA-UUC-GAG-GUA-ACG-CCC-
Problem 28-25
Which of the following base sequences would most likely be recognized by a restriction endonuclease? Explain.
(a) GAATTC
(b) GATTACA
(c) CTCGAG
Problem 28-26
For what amino acids do the following ribonucleotide triplets code?
(a)AAU
(b) GAG
(c) UCC
(d) CAU
Problem 28-27
From what DNA sequences were each of the mRNA codons in Problem 28-26 transcribed?
Problem 28-28
What anticodon sequences of tRNAs are coded for by the codons in Problem 28-26?
Problem 28-29
Draw the complete structure of the ribonucleotide codon UAC. For what amino acid does this sequence code?
Problem 28-30
Draw the complete structure of the deoxyribonucleotide sequence from which the mRNA codon in Problem 28-29 was transcribed.
Problem 28-31
Give an mRNA sequence that will code for the synthesis of metenkephalin. Tyr-Gly-Gly-Phe-Met
Problem 28-32
Give an mRNA sequence that will code for the synthesis of angiotensin II. Asp-Arg-Val-Tyr-Ile-His-Pro-Phe
Problem 28-33
What amino acid sequence is coded for by the following DNA coding strand (sense strand)? (5′) CTT-CGA-CCA-GAC-AGC-TTT (3′)
Problem 28-34
What amino acid sequence is coded for by the following mRNA base sequence? (5′) CUA-GAC-CGU-UCC-AAG-UGA (3′)
Problem 28-35
If the DNA coding sequence -CAA-CCG-GAT- were miscopied during replication and became
-CGA-CCG-GAT-, what effect would there be on the sequence of the protein produced?
Problem 28-36
Show the steps involved in a laboratory synthesis of the DNA fragment with the sequence CTAG.
Problem 28-37
Draw the structure of cyclic adenosine monophosphate (cAMP), a messenger involved in the regulation of glucose production in the body. Cyclic AMP has a phosphate ring connecting the 3′- and 5′-hydroxyl groups on adenosine.
Problem 28-38
Valganciclovir, marketed as Valcyte, is an antiviral agent used for the treatment of cytomegalovirus. Called a prodrug, valganciclovir is inactive by itself but is rapidly converted in the intestine by hydrolysis of its ester bond to produce an active drug, called ganciclovir, along with an amino acid.
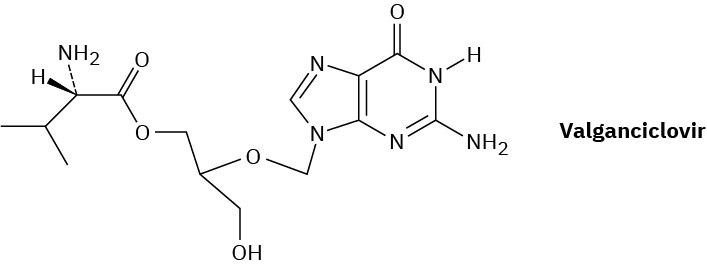
(a) What amino acid is produced by hydrolysis of the ester bond in valganciclovir?
(b) What is the structure of ganciclovir?
(c) What atoms present in the nucleotide deoxyguanine are missing from ganciclovir?
(d) What role do the atoms missing from deoxyguanine play in DNA replication?
(e) How might valganciclovir interfere with DNA synthesis?

