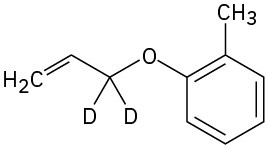
Visualizing Chemistry
Problem 30-13
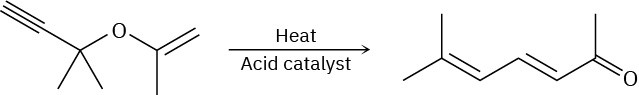
Predict the product obtained when the following substance is heated:
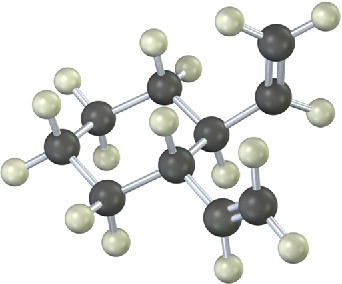
Problem 30-14
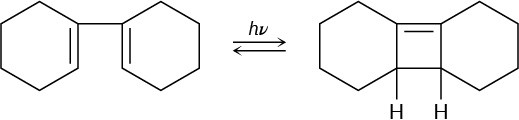
The 13C NMR spectrum of homotropilidene taken at room temperature shows only three peaks. Explain.
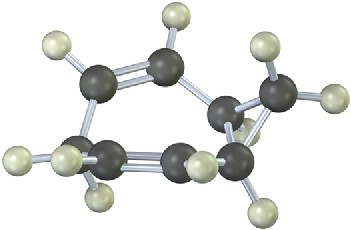
Mechanism Problems
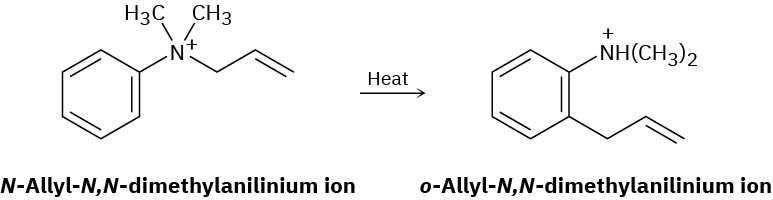
Problem 30-15
The following rearrangement of N-allyl-N,N-dimethylanilinium ion has been observed. Propose a mechanism.
Problem 30-16
Plastic photochromic sunglasses are based on the following reversible rearrangement of a dye inside the lenses that occurs when the lenses are exposed to sunlight. The original dye absorbs UV light but not visible light and is thus colorless, while the rearrangement product absorbs visible light and is thus darkened.
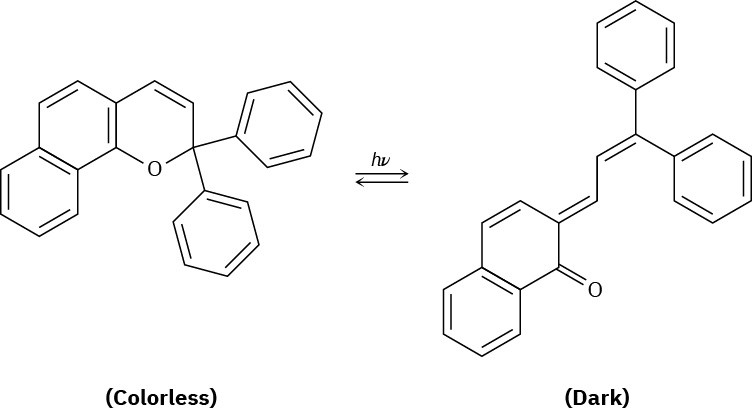
(a) Show the mechanism of the rearrangement.
(b) Why does the rearrangement product absorb at a longer wavelength (visible light) than the original dye (UV)?
Problem 30-17
The sex hormone estrone has been synthesized by a route that involves the following step. Identify the pericyclic reactions involved, and propose a mechanism.
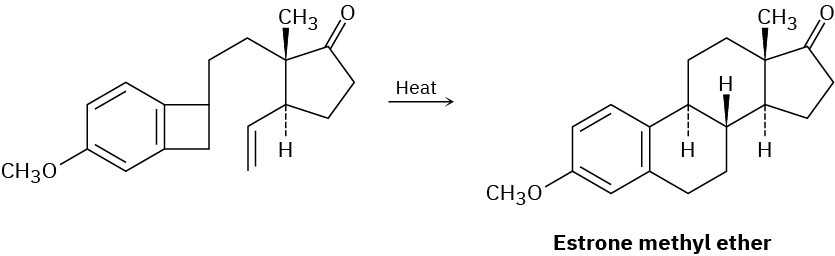
Problem 30-18
Coronafacic acid, a bacterial toxin, was synthesized using a key step that involves three sequential pericyclic reactions. Identify them, and propose a mechanism for the overall transformation. How would you complete the synthesis?
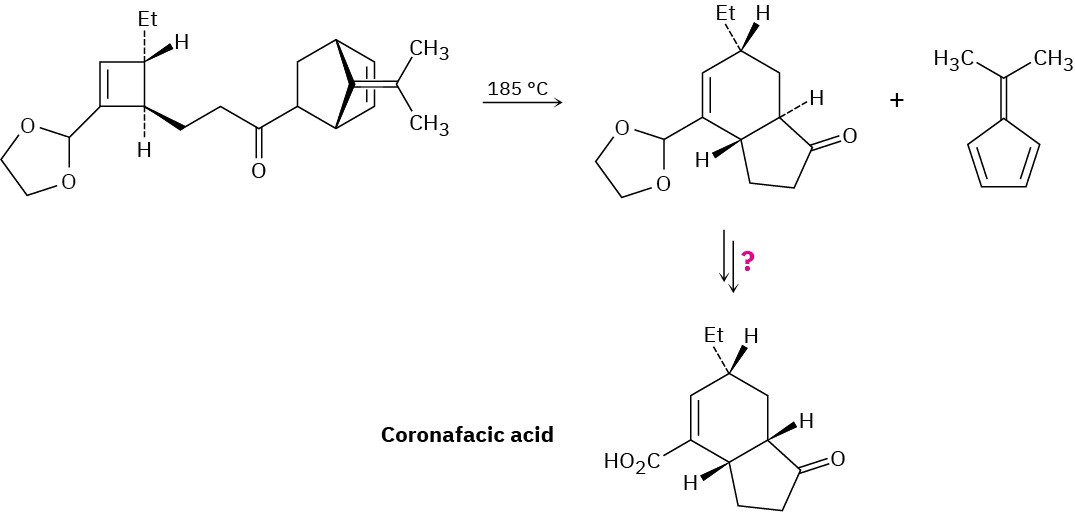
Problem 30-19
The following thermal rearrangement involves two pericyclic reactions in sequence. Identify them, and propose a mechanism to account for the observed result.
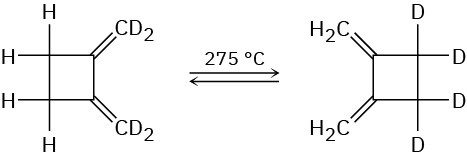
Electrocyclic Reactions
Problem 30-20
Do the following electrocyclic reactions take place in a conrotatory or disrotatory manner? Under what conditions, thermal or photochemical, would you carry out each reaction?
(a)
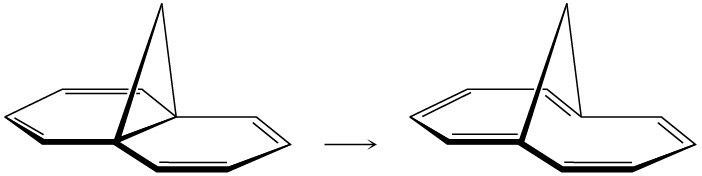
(b)
Problem 30-21
The following thermal isomerization occurs under relatively mild conditions. Identify the pericyclic reactions involved, and show how the rearrangement occurs.
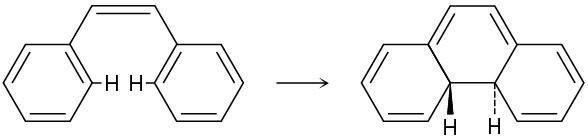
Problem 30-22
Would you expect the following reaction to proceed in a conrotatory or disrotatory manner? Show the stereochemistry of the cyclobutene product, and explain your answer.
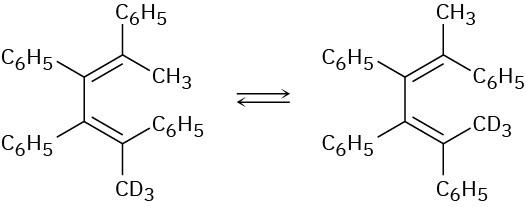
Problem 30-23
Heating (1Z,3Z,5Z)-1,3,5-cyclononatriene to 100 °C causes cyclization and formation of a bicyclic product. Is the reaction conrotatory or disrotatory? What is the stereochemical relationship of the two hydrogens at the ring junctions, cis or trans?
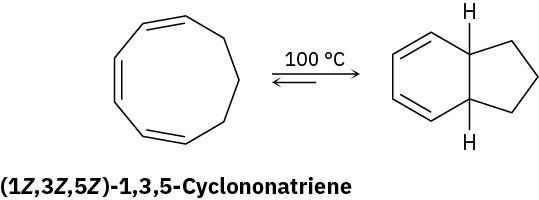
Problem 30-24
(2E,4Z,6Z,8E)-2,4,6,8-Decatetraene has been cyclized to give 7,8-dimethyl-1,3,5-cyclooctatriene. Predict the manner of ring-closure—conrotatory or disrotatory—for both thermal and photochemical reactions, and predict the stereochemistry of the product in each case.
Problem 30-25
Answer Problem 30-24 for the thermal and photochemical cyclizations of (2E,4Z,6Z,8Z)-2,4,6,8-decatetraene.
Problem 30-26
The cyclohexadecaoctaene shown isomerizes to two different isomers, depending on reaction conditions. Explain the observed results, and indicate whether each reaction is conrotatory or disrotatory.
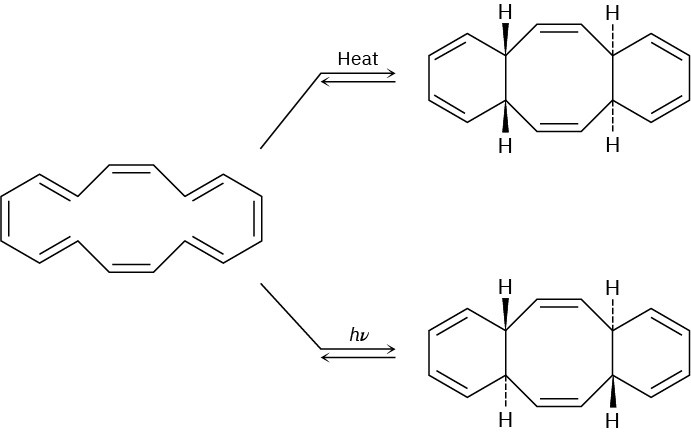
Cycloaddition Reactions
Problem 30-27
Which of the following reactions is more likely to occur? Explain.
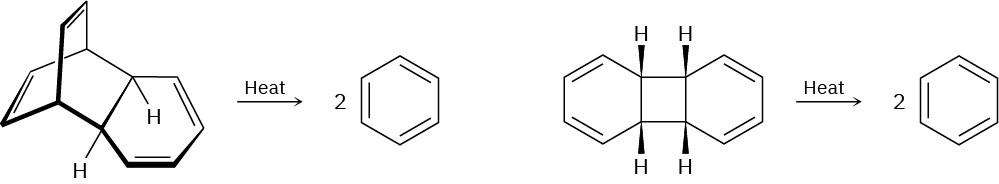
Problem 30-28
The following reaction takes place in two steps, one of which is a cycloaddition while the other is a reverse cycloaddition. Identify the two pericyclic reactions, and show how they occur.
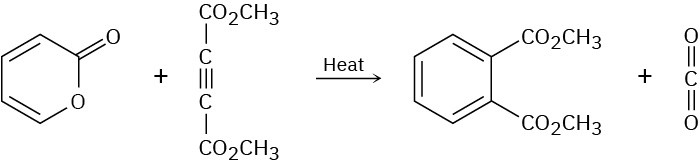
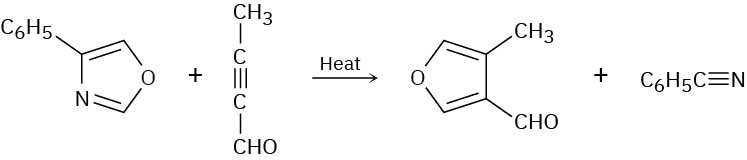
Problem 30-29
Two sequential pericyclic reactions are involved in the following furan synthesis. Identify them, and propose a mechanism for the transformation.
Sigmatropic Rearrangements
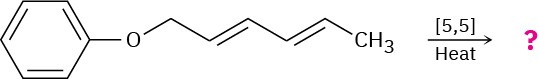
Problem 30-30
Predict the product of the following pericyclic reaction. Is this [5,5] shift a suprafacial or an antarafacial process?
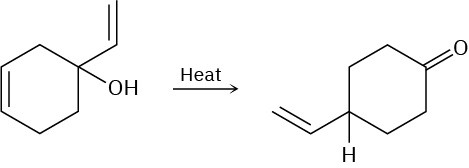
Problem 30-31
Propose a pericyclic mechanism to account for the following transformation:
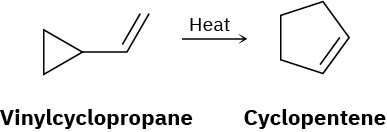
Problem 30-32
Vinyl-substituted cyclopropanes undergo thermal rearrangement to yield cyclopentenes. Propose a mechanism for the reaction, and identify the pericyclic process involved.
Problem 30-33
The following synthesis of dienones occurs readily. Propose a mechanism to account for the results, and identify the kind of pericyclic reaction involved.
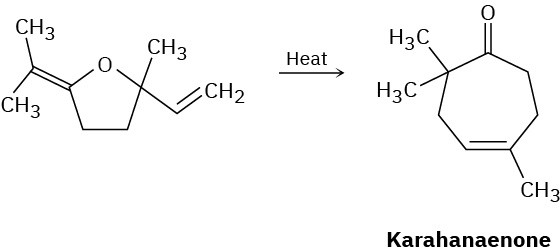
Problem 30-34
Karahanaenone, a terpenoid isolated from oil of hops, has been synthesized by the thermal reaction shown. Identify the kind of pericyclic reaction, and explain how karahanaenone is formed.
General Problems
Problem 30-35
What stereochemistry—antarafacial or suprafacial—would you expect to observe in the following reactions?
(a) A photochemical [1,5] sigmatropic rearrangement
(b) A thermal [4 + 6] cycloaddition
(c) A thermal [1,7] sigmatropic rearrangement
(d) A photochemical [2 + 6] cycloaddition
Problem 30-36
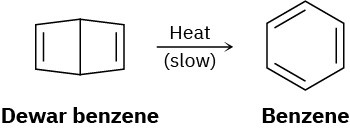
Bicyclohexadiene, also known as Dewar benzene, is extremely stable despite the fact that its rearrangement to benzene is energetically favored. Explain why the rearrangement is so slow.
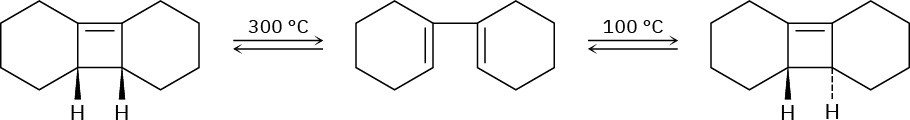
Problem 30-37
Ring-opening of the trans-cyclobutene isomer shown takes place at much lower temperature than a similar ring-opening of the cis-cyclobutene isomer. Explain the temperature effect, and identify the stereochemistry of each reaction as either conrotatory or disrotatory.
Problem 30-38
Photolysis of the cis-cyclobutene isomer in Problem 30-37 yields cis-cyclododecaen-7-yne, but photolysis of the trans isomer yields trans-cyclododecaen-7-yne. Explain these results, and identify the type and stereochemistry of the pericyclic reaction.
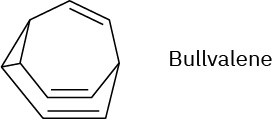
Problem 30-39
The 1H NMR spectrum of bullvalene at 100°C consists only of a single peak at 4.22 δ. Explain.
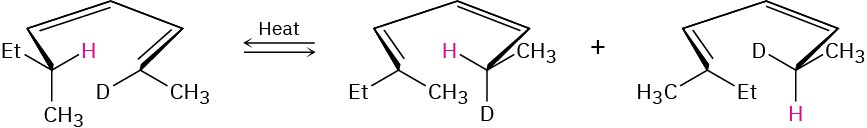
Problem 30-40
The following rearrangement was devised and carried out to prove the stereochemistry of [1,5] sigmatropic hydrogen shifts. Explain how the observed result confirms the predictions of orbital symmetry.
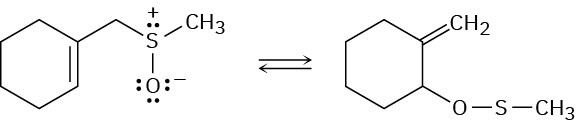
Problem 30-41
The following reaction is an example of a [2,3] sigmatropic rearrangement. Would you expect the reaction to be suprafacial or antarafacial? Explain.
Problem 30-42
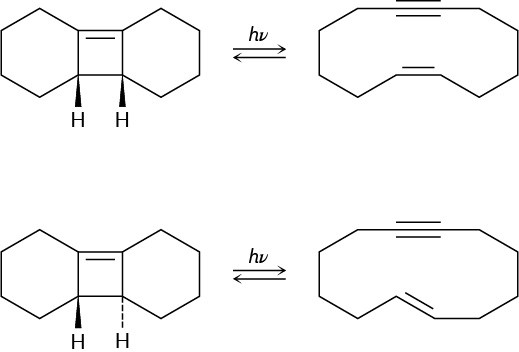
When the compound having a cyclobutene fused to a five-membered ring is heated, (1Z,3Z)-1,3-cycloheptadiene is formed. When the related compound having a cyclobutene fused to an eight-membered ring is heated, however, (1E,3Z)-1,3-cyclodecadiene is formed. Explain these results, and suggest a reason why opening of the eight-membered ring occurs at a lower temperature.
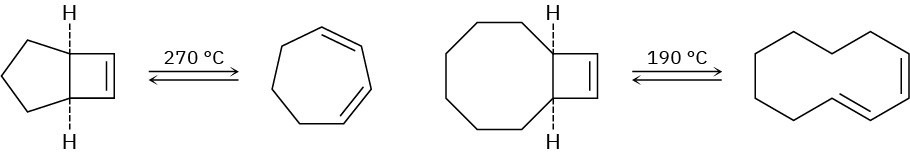
Problem 30-43
In light of your answer to Problem 30-42, explain why a mixture of products occurs in the following reaction:
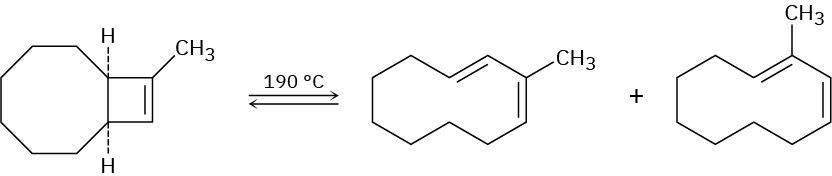
Problem 30-44
In nature, the enzyme chorismate mutase catalyzes a Claisen rearrangement of chorismate that involves both the terminal double bond and the double bond with the highlighted carbon. What is the structure of prephenate, the biological precursor to the amino acids phenylalanine and tyrosine?
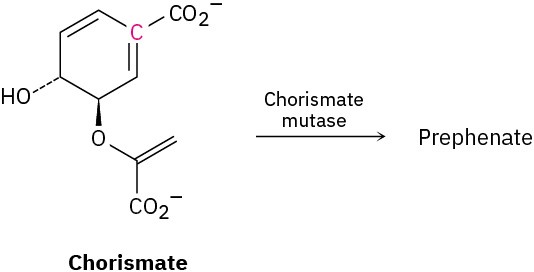
Problem 30-45
Predict the product(s) if the starting materials underwent a Claisen rearrangement. Draw arrows to illustrate the rearrangement of electrons.
(a)
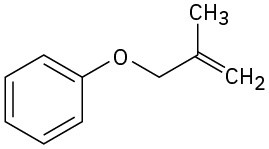
(b)
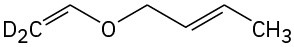
(c)